Encapsulating Synthetic STING Agonist, MSA-2, in Endosomolytic Polymersomes for Cancer Immunotherapy
ABSTRACT
The immune system response is a crucial mechanism in eliminating cancerous and damaged cells. The Stimulator of Interferon Genes (STING) protein is key to eliciting an immune response to cancer. Current STING-based immunotherapeutics (e.g., cGAMP) have poor pharmacokinetic properties and are restricted to intratumoral administration. Due to the poor drug-like properties of STING agonists, use of synthetic STING agonists has been heavily explored. A promising approach to remedying this limitation is the development of pH-responsive, endosomolytic nanocarriers which aim to enhance drug delivery to tumor sites by utilizing intracellular pH change. In this study, nanocarriers were characterized using Dynamic Light Scattering (DLS). Loading efficiency was quantified and nanocarrier treatments of varying drug concentrations were tested in cell assays. Uniform nanocarriers were produced; however, we could not show the complete disassembly of these nanocarriers. This likely led to incomplete administration of the entire drug cargo. MSA-2 encapsulated nanocarriers exhibited lower levels of STING agonism compared to MSA-2 free drug or empty nanocarriers. However, we achieved similar levels of agonism between the three trial groups. In addition, we achieved an encapsulation efficiency of ~61%. In conclusion, our findings show comparable STING activation by MSA-2 encapsulated nanocarriers despite poor nanocarrier disassembly. Further research is warranted as modification to nanocarrier disassembly parameters could optimize MSA-2 delivery, thereby eliciting a greater immune response.
INTRODUCTION.
In 2024, an estimated 2,001,140 new cases of cancer will be diagnosed in the United States and 611,720 people will die from the disease[1]. Cancer appears in various forms across diverse patient profiles. Current mainstream treatment options for cancer include radiation therapy, chemotherapy and surgical removal[1]. Unfortunately, each of these options present significant drawbacks in both efficacy and long term patient health. However, an emerging field in cancer treatment that mitigates adverse outcomes caused by other treatments is immunotherapy. Immunotherapies aim to elicit a response from the host immune system in fighting cancerous cells.
The stimulator of interferon genes (STING) protein plays a critical role in initiating an immune response and in the activation of cytotoxic CD8+ T-cells[3]. Additionally, activation of the STING protein in cells results in the increased release of type 1 interferons and release of proinflammatory cytokines[4]. For this reason, past research has manipulated this pathway with various drugs such as the naturally present STING agonist, 2′,5′-3’5′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP). While previous research has shown that treatment with cGAMP increases STING activation in cells[6], there are still many challenges in delivering drugs to cells, such as the short half-life of some drugs, poor endosomal escape, as well as inadequate cellular membrane permeability[6,7,8].
A proposed solution to these crucial challenges is the development of nanocarriers that act as vessels for pharmaceutical cargo [5,8]. Nanocarriers encapsulated with cGAMP have been shown to improve STING activation as well as reduce tumor volume [6]. A promising method to formulate such nanocarriers has also been documented in previous research. Flash nanoprecipitation (FNP) has been shown to be capable of producing various consistent iterations of (PEG)-EB nanocarriers [6]. A novel synthetic STING agonist has also been shown to activate the STING pathway while being amenable to oral administration [4,7]. MSA-2, presents a much more versatile and favorable drug choice for immunotherapies due to its easier method of administration. This research investigates the loading of MSA-2 into nanocarriers, quantifying STING agonism, and guiding the rational design of future immunotherapies.
MATERIALS AND METHODS.
Materials.
MSA-2 was synthesized following the protocol utilized by Pan et al. [4]. All other materials were sourced from Sigma Aldrich unless otherwise noted.
Chain Transfer Agent/Polymer Synthesis and Purification.
A 2 kDa (PEG)-functionalized reversible addition-fragmentation chain transfer polymerization-compatible chain transfer agent (PEG-2k-Ester-CTP) was synthesized via carbodiimide-mediated ester coupling with N,N’-Dicyclohexylcarbodiimide (DCC) and 4-(N,N-Dimethylamino) pyridine (DMAP) as a catalyst (Figure 1). PEG-2k-Ester-CTP was purified via precipitation in pentane and was subsequently dried under vacuum. Purified polymerization-compatible chain transfer agent (CTA) was used to facilitate the controlled radical copolymerization of 2-(Diethylamino) ethyl methacrylate (DEAEMA) and butyl methacrylate (BMA) at a 31:19 molar ratio relative to the CTA respectively. 4,4′-Azobis (4-cyanovaleric acid) (ACVA) was used as the free radical initiator (10:1 CTA:Initiator molar ratio) and dioxane was used as the solvent in this reaction. The polymerization was carried out in a 10 mL pear-shaped flask under inert conditions and was left to stir in a heated oil bath at 70 °C for 24 hours. After the polymerization, DEAEMA and BMA had a monomer conversion of 80% via H-NMR, yielding a degree of polymerization (DP) of 40 with an average of 25 DEAEMA and 15 BMA monomers incorporated in each polymer chain (Figure 1). The crude reaction mixture was dialyzed against acetone followed by water and was then lyophilized. While the CTA and polymer were successfully synthesized, the polymer did not perform as expected during initial attempts to assemble the nanocarriers. Therefore, for subsequent trials, we utilized an existing polymer batch which was extensively characterized and evaluated in previous research [6].
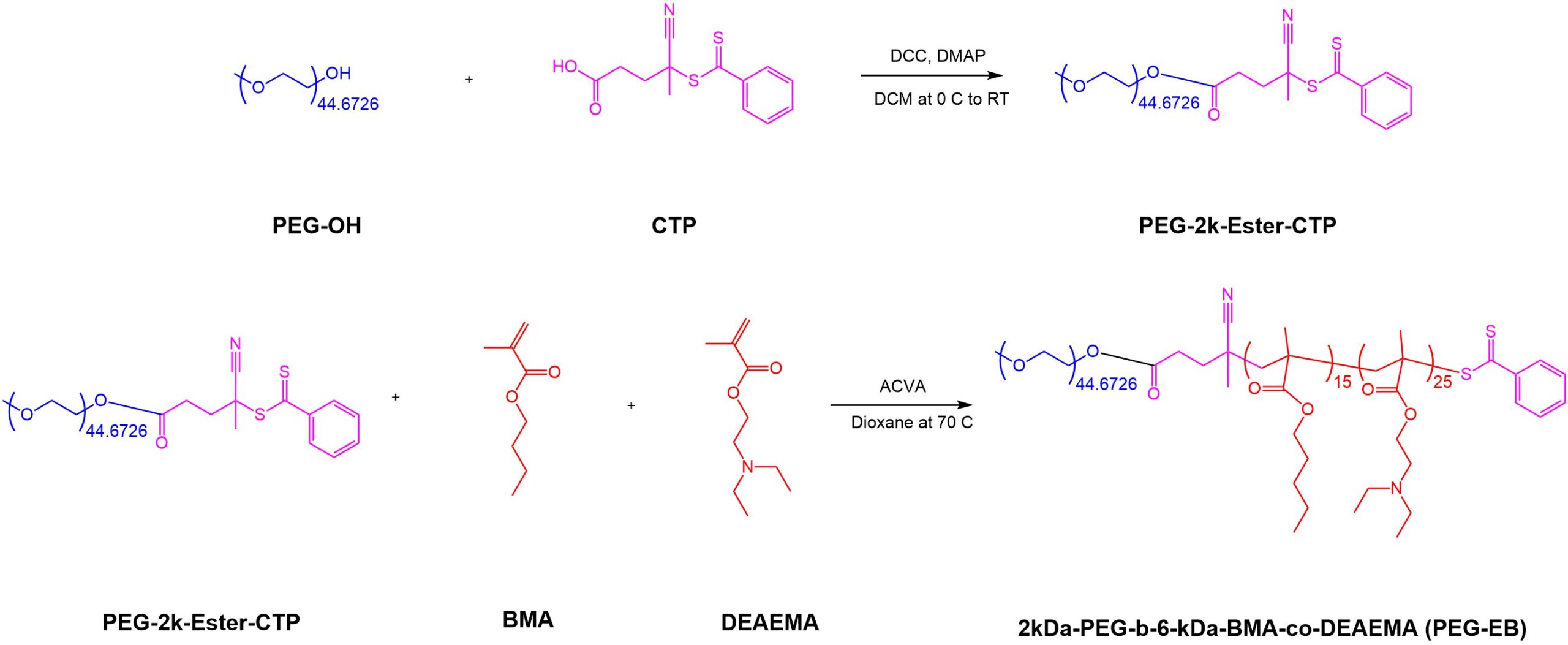
Polymeric Nanocarrier Formulation.
Polymeric nanocarriers were formulated using (FNP). In brief, FNP relies on an impingement device (Figure 2) to introduce two inlet liquid streams, an aqueous and an organic, to a small chamber where they experience turbulent mixing, forcing nanocarrier assembly and encapsulation of the hydrophobic cargo. In this research, PEG-EB and MSA-2 were dissolved in the organic stream to encourage MSA-2 loading in the hydrophobic section of the nanocarrier upon turbulent mixing. Five impingements were completed using 1 mL syringes. During the first impingement, the syringe for the aqueous inlet was loaded with 1 mL of deionized (DI) water, and the syringe for the organic stream contained PEG-EB and MSA-2 dissolved in tetrahydrofuran (THF) at 5 mg/mL each. Both syringes were rapidly pressed, forcing the liquids through the turbulent mixing chamber and into a 20 mL scintillation vial. Both syringes were filled equally with the remaining solution from the previous impingement for the next three impingements. During the last impingement, both syringes were equally filled with the solution from the previous impingement, however, after depressing both syringes, the solution went into a quench bath and was left to stir on a stir plate for 5 minutes. The quench bath was 4 mL of DI water.
Nanocarrier Filtration.
The contents of the quench bath from the nanocarrier formulation were loaded into centrifugal spin filters (Amicon 50k kDa MWCO). The nanoparticle suspension was centrifuged four times at 4700 xg for 25 minutes to eliminate unencapsulated cargo.
Nanocarrier Characterization.
DLS was used to analyze the hydrodynamic diameter of the nanocarrier, as well as nanoparticle disassembly in response to pH. In trials where nanocarrier formation was being confirmed and characterized, a stock concentration of nanocarrier was diluted 20x into pure DI water and loaded into semi-micro cuvettes. In trials where nanocarrier disassembly was being characterized, a stock concentration of nanocarrier was diluted 20x into phosphate-buffered solutions at varying pH values and loaded into semi-micro cuvettes.
QuantiLuc Assay.
A QuantiLuc assay was used to quantify STING agonism with MSA-2 encapsulated nanocarriers, as well as to compare STING agonism in nanocarriers against free MSA-2. THP-1 Duals are cells engineered to secrete an enzyme, luciferase, when the STING pathway is activated. QuantiLuc contains luciferin, which functions as the substrate for luciferase and, when luciferase binds to the substrate, it produces a quantifiable luminescence signal. Importantly, the QuantiLuc assay measures this luminescence relative to the other wells being tested in the same assay. Cells were dosed with MSA-2 encapsulated nanocarriers and free MSA-2 at concentrations of 250, 125, 62.5, 31.3, 15.6, 7.81, 3.91, 1.95 and 0.975 μM. Cells were also dosed with media alone to serve as a control for STING activation.
Quantifying MSA-2 Encapsulation.
High-performance liquid chromatography (HPLC) was used to quantify the loading of MSA-2 into nanocarriers. HPLC is able to identify individual molecular components by separating compounds by relative hydrophobicity, resulting in a unique elution time for each component. Various solutions with known concentrations of MSA-2 were also run on the HPLC, where the elution peaks were integrated, and a standard curve was formulated using these known concentrations. Based on this, a new sample could be loaded and the elution area could be plotted on the standard curve, which allowed for the concentration of MSA-2 in the sample to be back-calculated.
RESULTS.
Nanocarrier Formulation and Disassembly.
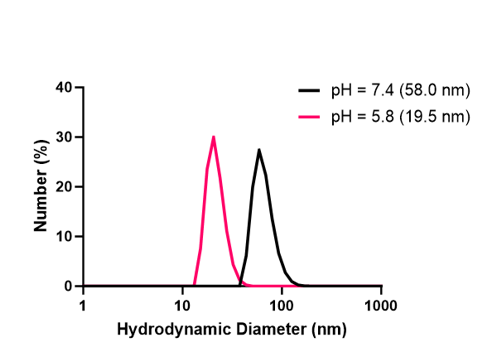
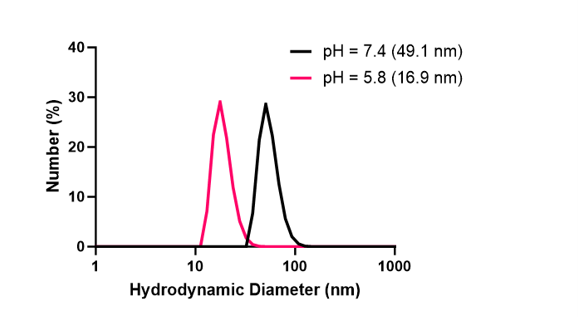
DLS readings showed that at a pH of 7.4, empty nanocarriers were forming uniformly. With optimal disassembly, at a pH of 5.0 all nanocarriers would completely disassemble into their polymeric building blocks, thus enabling the systemic release of all encapsulated drug cargo. While the nanocarriers were responsive to pH, it was unclear whether or not the nanocarriers were disassembling entirely into their polymeric building blocks (Figure 3). Similar trends were observed with MSA-2 encapsulated nanocarriers, however at a pH of 7.4, nanocarriers were forming with less uniformity (Figure 4).
MSA-2 Encapsulation.
Using the previously mentioned HPLC method, an encapsulation efficiency of around 61% was observed. Utilizing the HPLC trace shown (Figure 5), the area under each trial was calculated and used to form a standard curve (Figure 6). The unknown nanocarrier MSA-2 concentration was plotted against other known concentrations which allowed characterization of the unknown concentration. A concentration of around 12.18g/ml of MSA-2 was observed in the encapsulated nanocarriers.
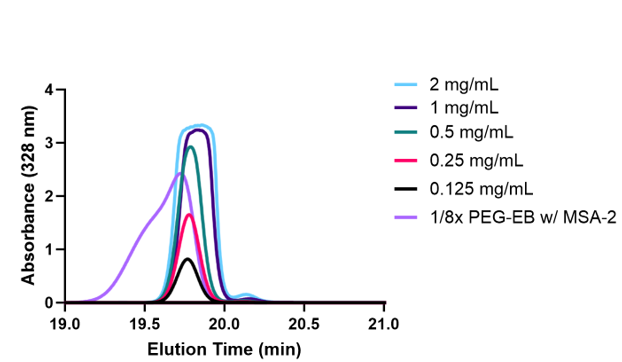
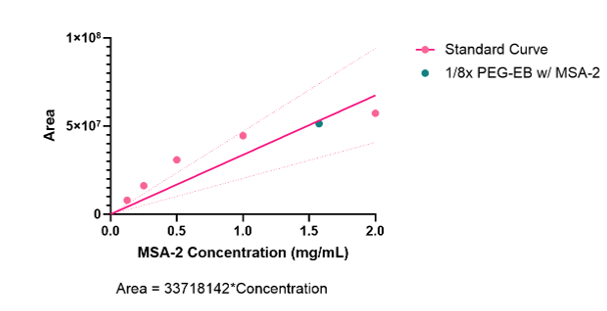
STING agonism.
In trials with THP-1 duals, it was observed that MSA-2 encapsulated nanocarriers could not beat levels of agonism achieved by MSA-2 free drug or by empty nanocarrier. The results of the QuantiLuc assay are included below (Figure 7).
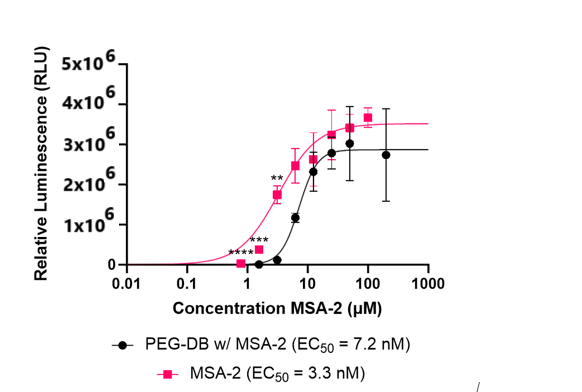
DISCUSSION AND CONCLUSION.
During this research, uniform nanocarriers were formed using flash nanoprecipitation, and were shown to be pH-responsive to a certain degree, showing distinct variations in size in response to pH. It is hypothesized that the inability of the nanocarriers to completely disassemble likely resulted in an incomplete administration of drug. This could be cited as a limitation of this research. However, this observation can also help explain some findings. Despite the incomplete disassembly of the nanocarriers, MSA-2 Encapsulated nanocarriers could provide similar STING agonism to that observed in free drug MSA-2 and empty nanocarriers. Future directions may include ensuring complete disassembly of nanocarriers, more detailed characterization on the effect of MSA-2 on the formation of nanocarriers as nanocarriers formed less uniformly when MSA-2 was encapsulated. Additionally, expanding testing to an in-vivo environment, as this could add factors that are hard to replicate in an in-vitro setting such as in the THP-1 Duals cell line. Some possible changes to methodology to achieve these goals include a modification to the polymer used to form the nanocarriers as well as a change to the method of release. Further research is warranted in this field as the development of pH responsive nanocarriers with the ability of loading various pharmaceuticals will improve the efficacy of future immunotherapies.
ACKNOWLEDGMENTS.
I would like to thank Jacob Schulman for his mentorship and guidance, Dr. John T. Wilson for the use of his lab as well as keen insight. Lastly, all the instructors at the School for Science and Math at Vanderbilt (SSMV) for their support and help in arranging my research internship.
REFERENCES
- National Cancer Institute. Cancer Statistics. National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/statistics (Accessed: May 9, 2024).
- Sompayrac, How the immune system works (Wiley-Blackwell, ed. 7, 2012).
- Shae, J. Baljon, M. Wehbe, P. Christov, K. Becker, A. Jumar, N. Suryadevara, C. Carson, C. Palmer, F. Knight, S. Joyce, J. Wilson. Co-delivery of Peptide Neoantigens and Stimulator of Interferon Genes Agonists Enhances Response to Cancer Vaccines, ACS Nano. 8, 9904-9916, (2020)
- Pan, S.A. Perera, J.A. Piesvaux, J.P. Presland, G.K. Schroeder, J.N. Cumming, B.W. Trotter, M.D. Altman, A.V. Buevich, B. Cash, S. Cemerski, W. Chang, Y. Chen, P.J. Dandiliker, G. Feng, A. Haidle, T. Henderson, J. Jewell, I. Kariv, I. Knemeyer, J. Kopinja, B.M Lacey, J. Laskey, C.A. Lesburg, R. Liang, B.J. Long, M. Lu, Y. Ma, E.C. Minnihan, G. O’Donnell, R. Otte, L. Price, L. Rakhilina, B. Sauvagnat, S. Sharma, S. Tyagarajan, H. Woo, D.F. Wyss, S. Xu, D.J. Bennett, G.H. Addona, An orally available non-nucleotide STING agonist with antitumor activity. Science 369, eaba6098(2020).
- Shams, A. Golchin, A. Azari, L. Amirabad, F. Zarein, A. Khosravi, A. Ardeshirylajimi. Nanotechnology-based products for cancer immunotherapy. Mol Biol Rep 49, 1389–1412 (2022).
- M. Pagendarm, P.T. Stone, B.R. Kimmel, J.J. Baljon, M.H. Aziz, L.E. Pastora, L. Hubert, E.W. Roth, S. Almunif, E.A. Scott, J.T. Wilson, Engineering endosomolytic nanocarriers of diverse morphologies using confined impingement jet mixing. Nanoscale 15, 16016-16029 (2023).
- Yang, X. Luo, J. Ma, Y. Wang, N. Cheng, A next-generation STING agonist MSA-2: From mechanism to application. Journal of Controlled Release 371, 273-287 (2024).
- D.J. Irvine, E.L. Dane, Enhancing cancer immunotherapy with nanomedicine, Nature Review Immunology 20, 321–334 (2020).
Posted by buchanle on Wednesday, June 25, 2025 in May 2025.
Tags: cancer, Flash nanoprecipitation, Immunotherapy, MSA-2, Nanocarrier, STING

