Optimization of Expression and Purification of an Anti-Inflammatory Binding Motif
ABSTRACT
Gevokizumab (Gevo) is a monoclonal antibody that binds strongly to IL-1β and blocks IL-1β receptor activation, effectively treating diseases with IL-1 imbalances. Antibody-based single-chain variable fragments (scFv) have a high affinity for the antigen target, making them useful molecular biology tools. This project aims to create a protocol to produce the Gevo scFv because it is thought that synthetic Gevokizumab scFV will be able to hinder the activation of IL-1β, which is a significant potent inducer of cartilage wear down. It is hypothesized that codon-optimization will allow for more production of scFv than with the wild-type gene (unoptimized) of the scFv Gevo. Plasmids were constructed by restriction enzyme digestion of the pET22b expression plasmid followed by HiFi assembly of a PCR insert, initially used to transform E. coli DH5a and E. coli BL21(DE3) for protein expression. Clones were validated by restriction enzyme digestion and colony PCR. Protein was expressed by growing bacteria at a large scale and then purification with a cobalt resin that binds to the his-tag on the target protein. A protein was purified near the expected band at 29KD on the codon-optimized samples of KS/BSB1, but the protein wasn’t purified from the wildtype Gevokizumab scFv. These results suggest that codon optimization improved protein production. The next step is to determine if the protein is biologically active. This research is significant as millions worldwide are affected by highly inflammatory diseases like osteoarthritis where IL-1β wears down the protective cartilage.
INTRODUCTION.
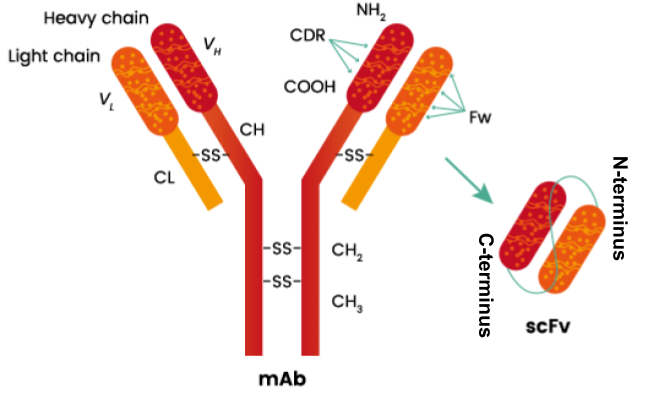
Osteoarthritis is an inflammatory disease where the cartilage that cushions joints to protect them starts to wear down over time [1]. This is caused by a known inflammatory receptor interleukin-1 beta (IL-1β), which is a potent inducer of cartilage wear down [2]. IL-1β is a cytokine involved in regulating immune and inflammatory responses [3]. By blocking IL-1β receptor activation, Gevokizumab, a monoclonal antibody that exhibits strong binding affinity to IL-1β, has the potential to treat diseases with IL-1 imbalances [4]. Due to its high affinity for the antigen target, Gevokizumab’s antibody-based single-chain variable fragments (scFv, Figure 1), which are the variable regions of the heavy (VH) and light chains (VL) of antibodies, connected with a linker peptide of amino acids, is a powerful molecular tool in molecular biology and therapeutic settings due to its broad purpose [5].
MATERIALS AND METHODS.
Plasmid Construction.
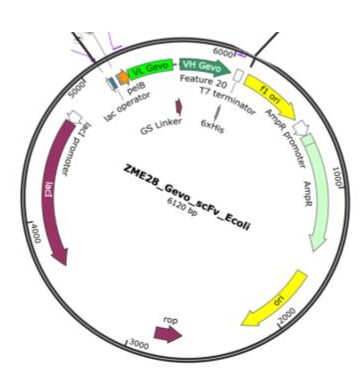
The sequence for the Gevokizumab (Gevo) scFV was cloned into a bacterial expression vector (pET22b), which contained a pelB leader sequence [6] and a His-tag sequence to facilitate protein expression. Plasmids were constructed using restriction enzyme digestion of a backbone bacteria expression vector. Enzymes BamH1 and Blp1 (NEB) with a rCutsmart (NEB) buffer were utilized according to the manufacturer-recommended procedure. The sequence encoding the Gevo scFv was synthesized (GeneArt, ThermoFisher) based on the published sequence (International Publication Date 4 January 2007 (04.01.2007) International Publication Number WO 2007/002261 A2) or a codon-optimized version based on the E. coli codon usage table (SnapGene). The final constructs were generated by NEBuilder® HiFi DNA Assembly and transformed into Champion™ DH5α DE3 E. coli for DNA production (Champion CC5202) and then subsequently Arctic Express BL21 (Agilent) for protein production. The sequence of the plasmids was verified by whole plasmid sequencing (Plasmasauraus).
Protein Expression and Purification.
To express protein, the codon-optimized sequence and wild-type plasmids were transformed into Arctic express BL21. For protein purification, 1-L cultures were grown in LB supplemented with ampicillin for 3 hours at 30℃ to an OD600 of 0.8 before induction with 0.1 mM IPTG. After the addition of IPTG, the cultures were grown for 18 hrs at 18℃ with shaking at 250 rpm. To isolate the periplasmic proteins, pelleted bacteria went through osmotic shock lysis by suspending the cell pellet in TES buffer (0.2 M Tris-HCl (pH 8.0); 0.5 M sucrose; 0.5 mM EDTA) followed by dilution 1: 4 with water. Before equilibrating the lysate with cobalt resin the NaCl concentration was adjusted to 0.15 M. After incubating the periplasmic sample with cobalt resin (HisPur™ Cobalt Resin, ThermoFisher 89964) to remove the His-tagged Gevo scFv, the resin was washed with three times with wash buffers (20 mM Tris-HCl, pH 8.0; 0.15 M NaCl; 10 mM imidazole) and then an elution buffer (20 mM Tris-HCL, pH 8.0; 0.15M NaCl; 260 mM imidazole-HCl). Samples were analyzed with an SDS-PAGE.
SDS-PAGE.
Protein fractions were run on a Bio-Rad Criterion Precast 4-20% reducing SDS PAGE gel in Tris/Glycine/SDS buffer (25 mM Tris, pH 8.3; 192 mM glycine; 0.1% SDS) at 100V( constant voltage) until the dye reaches the bottom of the gel.
RESULTS.
To confirm that cloning was successful, 6 samples were sent off for the whole plasmid sequence. Four samples were from the wild-type clone (“ZME28”) and two samples were codon-optimized (“KS/BSB1”). From the data returned, it was determined that Clone 7 of ZME28 had a point mutation in the C-terminal HisTag region; therefore, the clone was discarded. It was also found that all clones had 3 mutations in the backbone compared to the published sequence. The mutations were not in areas that were important for this project’s focus on the scFv protein production. Sequencing showed that KS/BSB1-4 and KS/BSB1-5 were codon-optimized in the Gevokizumab scFv. Clone 5 of ZME28 (wildtype) and Clone 4 of KS/BSB1(codon optimized) were used for protein production as they had the highest plasmid DNA yield.
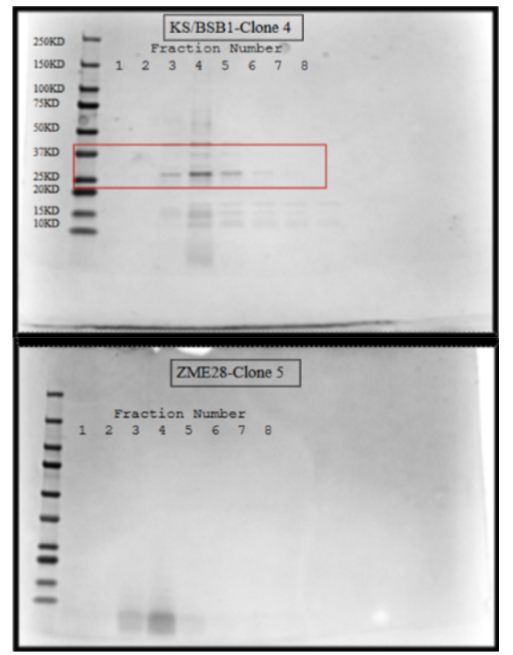
After running an SDS-PAGE gel it was observed that a protein was purified between 37kD and 25kD (figure 3), the expected band was at 29kD on the codon-optimized samples of KS/BSB1. Since this purification only utilized the cobalt column, contaminating proteins were also present in the optimized sample. While these bacterial proteins are concerning, we would need to further purify the sample, especially if endotoxin is present. No protein was purified in the wild-type clone of ZME28.
| Table 1. Whole plasmid sequence analysis comparing independently isolated clones of ZME28 and KS/BSB1. 4 clones of ZME28 (clone 4, 5, 7, 9) and 2 clones of KS/BSB1 (clone 4, 5) were sequenced. ZME28 consists of the normal scFv GEVO VL-VH fragment and KS/BSB1 consists of the codon-optimized scFv. Codon CGC(Arg) is preferred for E. Coli over AGA. The base pair changes made for codon optimization are highlighted in red. | |
| Original V-L Chain Sequence (ZME28) | Original V-H Chain Sequence (ZME28) |
| CCCGGCA | AGACAGCCCTCAG |
| Modified V-L Chain Sequence (BSB1) | Modified V-H Chain Sequence (BSB1) |
| CCGGGCA | CGCCAGCCGTCAG |
DISCUSSION.
This project aimed to create a protocol to produce the gevokizumab scFv as it is thought that synthetic gevokizumab scFV will be able to slow or stop the activation of IL-1β, which is a significant potent inducer of cartilage wear and tear. It had been hypothesized that codon-optimized gevokizumab would produce a greater amount of the gevokizumab scFv.
As shown in Table 1, the Gevokizumab scFv was optimized while the unoptimized version did not have these changes. All constructs that were sequenced had their respective sequence (codon optimized/wildtype) and were what was expected. It was found that all constructs had a mutation in the backbone. This wasn’t important as it had no impact on protein purification.
As shown in Figure 3, it was observed that a band was purified between 37KD and 25KD in the codon-optimized model. This is significant because the expected band was 29KD. It was also found that no protein was purified in the wild-type model, which we hypothesized was due to the lack of bacterial-optimized codons [7]. This suggested that the newly constructed codon-optimized plasmid produced robust gevokizumab scFv. This is novel as no other research has taken the Gevo scFv and used it in this way. Research has suggested codon optimizations and certain applications for gevokizumab, but not taking the scFv, optimizing it and then producing it.
Codon optimization does improve the protein production yield. Other research suggests using less frequently used codons lowers the production yield due to ribosome stalling and failed translation [7]. Other research has used a scFv [7, 8], but none have used a gevokizumab scFv. It is currently unknown if the purified gevokizumab with the optimized codon is biologically active, so the next steps will be to test its activity and usefulness in different models.
The project aims to move towards proving if the purified protein is biologically active. The plans to do this are to take the produced Gevokizumab scFv and compare the levels of mKate2 fluorescence which is activated by the amount of IL-1β produced and see if IL-1β lowers the amount of expression similarly to the lab-produced full antibody that was bought from the company that makes it (Thermofischer). A protocol to produce the gevokizumab scFv was created to produce the Gevokizumab scFv. Gevokizumab is a potent inhibitor of IL-1β. Since IL-1β is a potent inducer of cartilage degradation, Gevokizumab has potential significance in clinical trials of osteoarthritis treatment, and synthetic production of an optimized protein product, as created here and now under testing, could be instrumental as a treatment option.
ACKNOWLEDGMENTS.
I would like to acknowledge everyone who has helped me along the way. I would like to acknowledge Dr. Brunger for allowing me to have this opportunity, Becky for being an amazing mentor guide, Dr. Eeds for being a wonderful advisor, and my girls for being a great support system. I would also like to acknowledge my parents, Dad for driving me to my lab daily to conduct this research and Mom for listening to all of my presentation practices and any tangents I went on even though you didn’t understand any of it.
REFERENCES
- Mayo Clinic ”Osteoarthritis” Mayo Clinic, (2021). https://www.mayoclinic.org/diseases-conditions/osteoarthritis/symptoms-causes/syc-20351925.
- T.L. Vincent “IL-1 in osteoarthritis: time for a critical review of the literature” F1000Research 8, 934. (2019).
- H. Issafras, et. al., “Detailed Mechanistic Analysis of Gevokizumab, an Allosteric Anti–IL-1βAntibody with Differential Receptor-Modulating Properties.” Journal of Pharmacology and Experimental Therapeutics 348, 202–215 (2013).
- A. Owyang, et. al., “XOMA 052, an Anti-IL-1β Monoclonal Antibody, Improves Glucose Control and β-Cell Function in the Diet-Induced Obesity Mouse Model.” Endocrinology 151, 2515–2527 (2010).
- Z. Ahmed, et. al., “scFv Antibody: Principles and Clinical Application” Journal of Immunology Research 10.1155/2012/980250 (2012)
- R. Pasupuleti, et. al., “Genetic code expansion in E. coli enables production of a functional “ready-to-click” T cell receptor-specific scFv.” New Biotechnology 76, 127–137 (2023).
- A Tiwari, et. al., “Enhanced periplasmic expression of high affinity humanized scFv against Hepatitis B surface antigen by codon optimization. Protein Expression and Purification.” Protein Expression and Purification 74, 272–279 (2010).
- S.K. Sharma, et. al., “Improved soluble expression of a single-chain antibody fragment in E. coli for targeting CA125 in epithelial ovarian cancer.” Protein Expression and Purification 102, 27–37 (2014).
Posted by buchanle on Wednesday, June 25, 2025 in May 2025.
Tags: Gevokizumab, Inflammatory, Osteoarthritis, Protein Expression, scFv

