The Effect of Microplastic Pollution on Symbiotic Bacteria within the Marine Sponge, Halichondria panicea
ABSTRACT
Current research has shown that marine sponges are capable of synthesizing highly specific and effective biomedical compounds. These molecules are vital to the pharmaceutical industry, as many novel drugs are derived from preexisting antibacterial compounds found in nature. Currently, microplastic pollution is threatening the future of many marine organisms, including marine sponges. If enough of the sponge population is harmed, then future pharmaceutical discoveries may be limited or lost indefinitely. Therefore, this paper looks at the effect microplastics pollution can have on symbiotic bacterial communities living within the sponge, Halichondria panicea. To simulate pollution, specimens of the marine sponge, Halichondria panicea, were artificially introduced to environments with varying levels of microplastics for the duration of two weeks; the sponges were then homogenized, and its extracts incubated on petri dishes to determine the number of viable bacterial colonies after being exposed to a microplastic infested environment. The data recovered from the experiment were analyzed, subjected to a one-way ANOVA test, and utilized to derive a correlation coefficient. These data analysis approaches showed that the viability of symbiotic bacteria within the marine sponge, Halichondria panicea, is not necessarily affected by increases in microplastic pollution.
INTRODUCTION.
Marine microplastics are synthetic plastic polymers less than 5mm in diameter. These plastics are commonly mistaken as food and are unknowingly ingested by many organisms. Not only do they cause harm to the organism’s digestive tract, but they can also alter feeding behavior, affecting growth and reproductive cycles [4]. Microplastics are especially harmful to higher trophic organisms as they are most affected by environmental phenomena, biological magnification [1]. The concentration of microplastics within the marine environment is a heavily debated topic among marine biologists. However, a recent and predominant study from leading researcher Jennifer A. Brandon claims that there are about 8.3 million individual microplastics per cubic meter of ocean water [2]. This research finding was utilized to simulate ocean water for this particular project. The most common type of marine microplastic polluting Earth’s oceans is Polyethylene Terephthalate (PET). PET is non-biodegradable and can take up to 450 years to fully decompose in marine environments [3].
Within the phylum Porifera, there are many species of marine sponges that are essential for the viability of reef and other marine ecosystems. These sponges play a crucial role in promoting biodiversity and removing harmful particles from ambient waters. Because of their physiology, marine sponges can move water through their bodies at rates up to 35 mL/min*cm3 per cubic centimeter of sponge. With these flow rates, they can remove up to 90% of dissolved organic carbon and 95% of bacteria and other particles within their ambient environment [4].
As a result of the high rate of water movement, marine sponges are often in contact with an endless stream of virulent pathogens, making them a primary place to look for antibacterial compounds. Sponges attain these medical compounds through their symbiotic relationship with certain bacteria. Many times, when these bacteria are stressed, they produce compounds called secondary metabolites. These molecules are highly specialized and synthesized by bacteria to target specific problems [5]. In the case of the marine sponge, Halichondria panicea, current research has shown that there are a variety of bacteria living within the organism. However, its main symbiotic bacteria belong to the genus Rhodobacter (Proteobacteria, subdivision α). These bacteria are relatively small, 0.6-0.8 microns long, gram-negative, and possess immense potentials in the pharmaceutical industry as they are able to synthesize highly complex antibiotics and drugs [6].
When sponges move large amounts of water, microplastics often become logged in their pores as they traverse through the sponge’s body. This has caused many researchers to investigate the effects of pollution of marine sponges. Due to the marine sponges’ primitive physiology, the general consensus is that they are not usually affected by changes in water quality, including microplastic pollution [7]. The specific effects of microplastic pollution on symbiotic bacteria within sponges, however, have not been researched or examined [8]. Therefore, this paper focuses on a research area that is lacking in many prominent studies. Understanding how microplastic pollution may affect symbiotic bacteria is the first step towards comprehending what possible challenges may lie in the future as it relates to the viability of marine sponges and the pharmaceutical industry.
MATERIALS AND METHODS.
Materials.
The sponges used in this study were collected from boat docks near the Cabrillo Marine Aquarium and identified as specimens of the sponge, Halichondria panicea, by aquarium curators. The microplastic particles were 300-350 microns in diameter and acquired from the aquarium. All other tools including aquarium tanks, artificial seawater, heaters, lighting, marine sponge food, and filters, were borrowed from the local fish store.
Sponge Husbandry.
The sponges were placed into separate two-gallon tanks with LED lighting suspended above the tank, cycling at 12-hour intervals. Each tank also had a heater set at 23 °C, a Fluval AquaClear Power Filter with activated charcoal and sponge filtration media, and a five-watt Hydor Koralia Circulation pump. The sponges were spot-fed once a day with 5ml of Brightwell Aquatic’s PhytoGreen-M phytoplankton suspension. A 10% water change was also conducted on the tanks every three days for the duration of the two-week testing period.
Methods.
About four cubic centimeters of Halichondria panicea sponge was collected from boat docks near the Cabrillo Marine Aquarium. The sponges were then separated into single cubic centimeter pieces, cleaned, acclimated for artificial seawater, and then placed into separate tanks labeled, control, 0.07mL, 0.15mL, and 0.30mL. In the control tank, no artificial microplastics were added; in the 0.07 tank, 0.07 mL of microplastics were added per 100 mL of water; in the 0.15 tank, 0.15 mL of microplastics were added per 100 mL of water; in the 0.30 tank, 0.30 mL of microplastics were added per 100 mL of water as shown in Table 1. Each tank used a total of 5.0 L of artificial seawater. After two weeks, the sponges were cleaned by manually removing debris with scapples, and the tissue was homogenized using a pestle and mortar. The extracts were then filtered through a 1 µm syringe filter, diluted by a factor of 10:2 with artificial seawater, and inoculated onto Hektoen enteric agar plates to select for Rhodobacter for 48 hours at 25 °C with a 100 µL micropipette. After the incubation, the number of colonies on each plate was counted three times and averaged (to ensure consistency) and recorded as data. This process was repeated three more times as separate trials for a total of four separate trials.
Table 1. Total amount of microplastics added for each treatment group.
| Control | 0.07 | 0.15 | 0.30 | |
| Trial 1 | 0 | 3.5mL | 7.5mL | 15mL |
| Trial 2 | 0 | 3.5mL | 7.5mL | 15mL |
| Trial 3 | 0 | 3.5mL | 7.5mL | 15mL |
| Trial 4 | 0 | 3.5mL | 7.5mL | 15mL |
Table 2. Results showing the number of viable bacteria colonies from each treatment group.
| Control | 0.07 | 0.15 | 0.30 | |
| Trial 1 | 75 | 77 | 81 | 80 |
| Trial 2 | 83 | 82 | 70 | 73 |
| Trial 3 | 85 | 69 | 65 | 65 |
| Trial 4 | 70 | 80 | 62 | 74 |
RESULTS
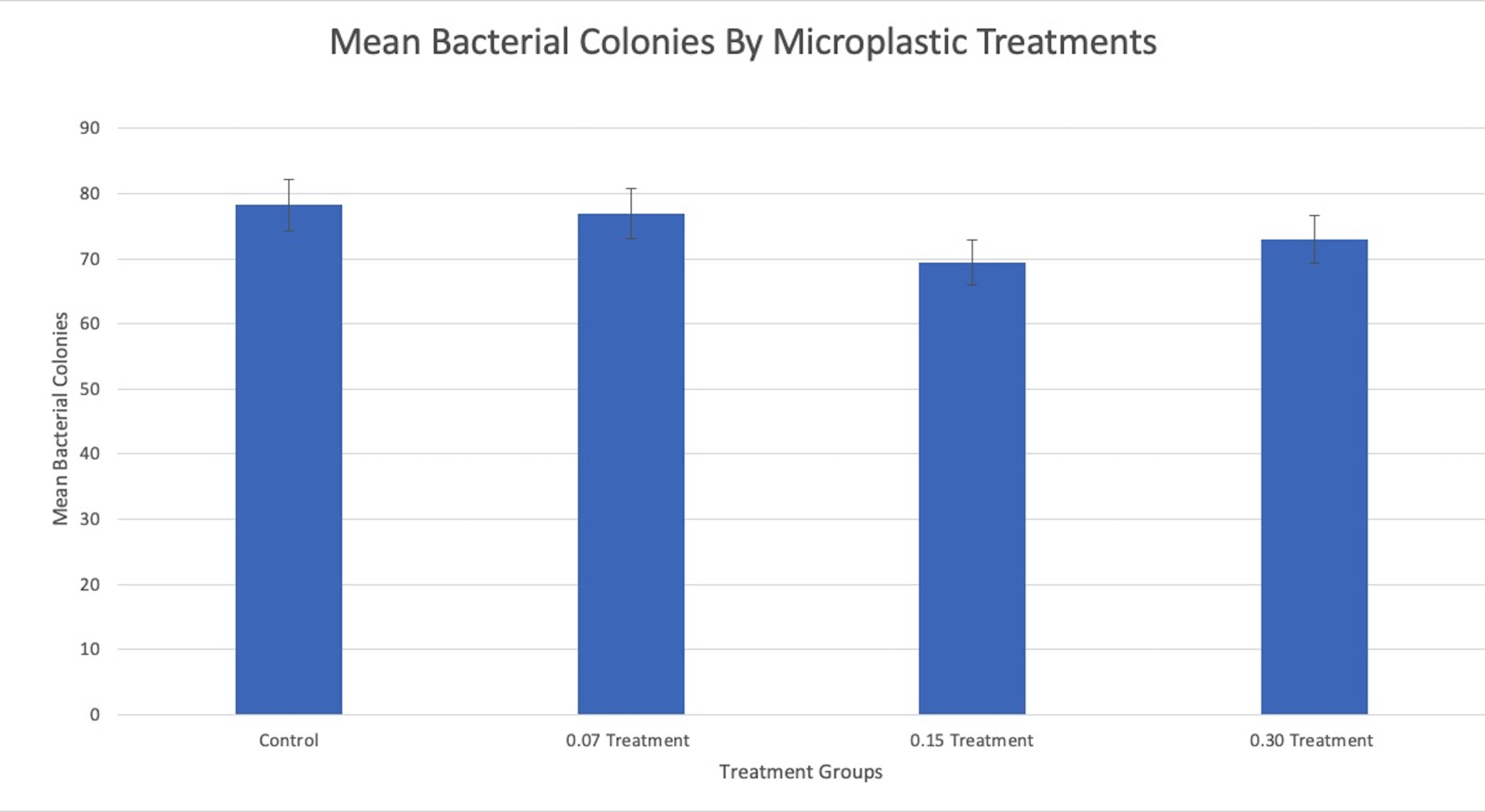
The number of viable bacterial colonies from each treatment is shown in Table 2. A one-way ANOVA test was performed on the raw data utilizing Excel Spreadsheets and yielded a P-value of 0.308, graphed in Figure 1. After the initial significance value was determined, a correlation coefficient test was also conducted on the data, resulting in a P-value of 0.377. Both these tests concluded that there is very little correlation between the number of viable bacterial colonies and the severity of microplastic pollution. In addition, the one-way ANOVA showed that there is no significant difference in the outcomes from any of the differing microplastic treatments.
Figure 1. Graphed results from the one-way ANOVA statical test showing the average number of viable bacteria colonies found on agar dishes after each microplastic treatment. There is no significant relationship between the average number of viable colonies and the concentration of microplastics in the sponges (P = 0.308).
DISCUSSION/CONCLUSION
The statistical analysis results show that microplastic pollution does not seem to affect the viability of bacteria living within the marine sponge, Halichondria panicea, and therefore may not affect the discovery of future pharmaceutical drugs. Steps were taken to ensure a greater survival probability of that bacteria from the genus, Rhodobacter, on the agar plates including filtration of crude extracts, and growth medium composition. Even with such steps, the bacteria grown on the dishes could not be determined and identified as specimens from any distinct species. The survivability of bacteria shown in this research generally align with current trends, as it reveals that marine sponges are relatively resilient organisms that can transcend many of the adverse effects caused by humanity’s disregard for the environment [9]. Although there is a plethora of research on drugs and pharmaceuticals derived from marine sponges, research regarding the relationship between marine sponge bacteria and its environmental factors is lacking. What little research is available, however, has shown that sponge bacteria are relatively resilient to changes in water chemistry when compared to their counterparts [10]. The reason for this phenomenon is yet to be researched and holds immense potential for the understanding of bacterial physiology in the future. However, possible pathways to explore the resiliency of sponge bacteria may include exposing certain bacteria strains to heavy metals or acidic environments. In these experiments, researchers can model the effects of pollution and climate change on the viability of marine sponge-associated bacteria.
ACKNOWLEDGMENTS.
I would like to thank my local fish store and aquarium for loaning my supplies and giving advice on how to care for the sponges. I also express sincere gratitude to the curators at the Cabrillo Marine Aquarium for their experience and guidance during my research.
REFERENCES
- Chatterjee, S. Sharma, Microplastics in our oceans and marine health. Field Actions Science Reports. 19, 54-61 (2019).
- A. Brandon, A. Freibott, L. M.Sala, Patterns of suspended and salp‐ingested microplastic debris in the North Pacific investigated with epifluorescence microscopy. Limn. and Ocean. Lett. 5, (2019).
- Cho, What Happens to All That Plastic? – State of the Planet. Our Oceans: A Plastic Soup. State of the Planet. (2019).
- M. Morganti, M. Ribes, G. Yahel, R. Coma, Size Is the Major Determinant of Pumping Rates in Marine Sponges. Front. in Phys. 10, 1474 (2019).
- Amade, D. Pesando, L. Chevrolet, Antimicrobial Activities of Marine Sponges from French Polynesia and Brittany. Biol. 70, 223–228 (1982).
- Althoff, C. Schütt, R. Steffen, R. Batel, W. E. GMüller, Evidence for a Symbiosis between Bacteria of the Genus Rhodobacter and the Marine Sponge Halichondria Panicea : Harbor Also for Putatively Toxic Bacteria? Biol. 130 529–536 (1998).
- J. Bell, H. M. Bennett, A. Rovellini, N. S. Webster, Sponges to Be Winners under Near-Future Climate Scenarios. BioScience. 68, 955-968 (2018).
- Fan, et. al. m, Marine microbial symbiosis heats up: The phylogenetic and functional response of a sponge holobiont to thermal stress. ISME. 7, 991-1002 (2013).
- Kelmo, J. J. Bell, M. J. Attrill, Tolerance of Sponge Assemblages to Temperature Anomalies: Resilience and Proliferation of Sponges following the 1997–8 El-Niño Southern Oscillation. PLOS ONE. 13, (2013).
- Anjum, et. al. Marine Sponges as a Drug Treasure. Biomol Ther (Seoul). 24, 347-362 (2016).
Posted by John Lee on Thursday, May 20, 2021 in May 2021.
Tags: Marine Biology, Marine Sponges, Microplastics, Symbiotic Bacteria