Survival of Tribolium confusum when co-infected with gregarine parasite at different life stages and Bacillus thuringiensis infection
ABSTRACT
Co-infection with multiple threats can pose a serious public health risk in humans and model species. Specific co-infections can either improve or harm immune responses. Using the model of Tribolium confusum, survival after co-infection between naturally infected eugregarine parasites, and a bacterial pathogen, B. thuringiensis, was predicted to decrease compared to groups with infection. Insects were infected with parasites at the larval stage and then disinfected, and parasites were readministered at the adult stage. Survival rates were measured after a septic stabbing was conducted on T. confusum with B. thuringiensis (low and medium infection dose). At both infection doses, adult beetles showed insignificant differences in survival post-infection between co-infected and singularly infected populations. Overall, our results suggest that bacterial infection with a medium dose (lethal dose 50) impacts confusum beetles’ survival with and without gregarine parasites at larvae and adult life stages. Further, we aim to determine the outcomes of co-infection between eugregarine parasites and B. thuringiensis on beetle survival. The outcome of co-infection has still not been found to change the health of the model organism and may have further health and fitness consequences such as increased immune resistance or decreased ability to tolerate infection-associated threats.
INTRODUCTION.
Compounding immunological threats can damage human and animal populations. Naturally occurring infections of bacteria, parasites, and viruses simultaneously suppress immunity within a host. It has been shown that multiple strains of HIV increase the symptoms and progression toward AIDS [1]. The co-infection of dengue fever and Zika virus are difficult to trace and can also lead to worsened symptoms. [1] A study in western Ethiopia found that 74.5% of the sample population was infected with dangerous parasites and viruses, like Schistosoma mansoni, asymptomatic Plasmodium falciparum malaria, and HBV and 16.6% were coinfected with multiple strains or infections. [2] Other studies have found that early-life infection can increase immune response and protect against biological threats more effectively [1]. In co-infection studies among varying model organisms, 8 out of 18 showed a rise in mortality due to co-infection [3]. Co-infection results often vary depending on the infection and host species and raise a serious question for widespread organismal health and immunity.
To study co-infection in a controlled environment, model organisms allow for the application of basic research into human health. Invertebrates are often used for their quick reproduction and relatively simple anatomy. While the findings of exact immune threats can not be applied to human subjects, the effects of harmful infections can be similar. If two symptomatically similar threats are present in both invertebrates and humans, it is beneficial to see how the model organisms react to hypothesize on connections between co-infection in humans. While model organisms can be given controlled infections, humans cannot and are much more difficult to trace. Often different strains of Hepatitis can co-infect one person but are untraceable. [1] Model organisms allow for controlled infection with uniform dosages and definite present threats.
This study aims to research the co-infection of immune-compromising parasite infestation and bacterial infection within a lab-cultured invertebrate host. Tribolium confusum (or confused flour beetle), is a viable species for lab study due to its recognition as a global pest, efficacy within evolutionary and immune studies, and similarities between a lab environment and natural habitat.[4] This study tests the comparison of co-infection of eugregarine parasites at different times of exposure in the organism’s life. We aim to find how parasites affect the survival of these invertebrates with a bacterium that has been shown to kill most invertebrate pests with a large dosage. We used alternate dosages for the bacterial infection to test the sensitivity of beetle survival between the co-infectants. We predict that there will be higher mortality for bacteria-infected organisms and that a higher prevalence of parasitic exposure in the early life stages will lower their ability to survive as an adult when exposed to bacteria.
MATERIALS AND METHODS.
Description of T. confusum. This study used two generations of the Tribolium confusum or confused flour beetle to investigate the host’s survival when early and late-life parasitic infection was compounded with bacterial exposure. We conducted two trials of this experiment from the same egg-laying population two months apart. The lab populations were descendants of wild-caught flour beetles from grain storage facilities. T. confusum has a life cycle of approximately five weeks and matures through five major life stages. Virgin females have been shown to have stronger fitness compared to mated females and males due to a stronger biological drive to survive and reproduce. [6] This study monitored virgin females from uncontaminated lab stocks of T. confusum throughout their life cycle.
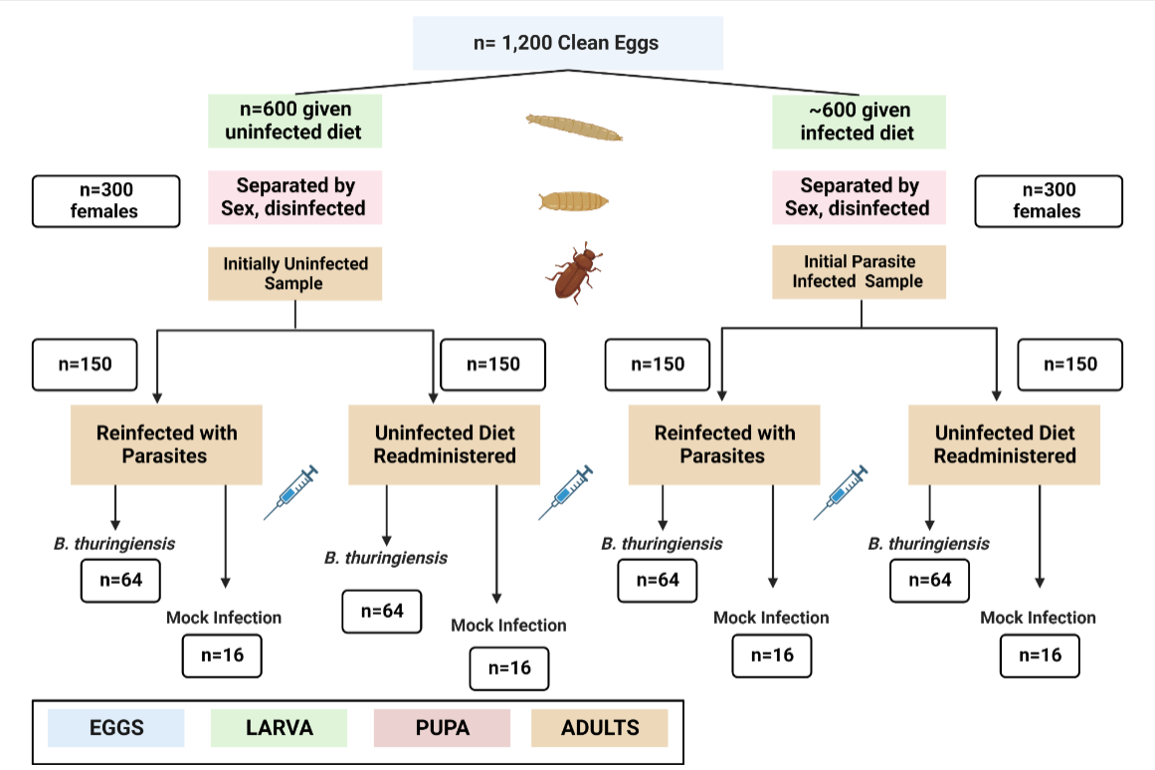
Collection and Initial Parasitic Infection. Roughly 1,200 eggs were collected 2-3 days apart. These eggs were harvested from adults that had no interaction with the eugregarine parasites. Each sample population was kept at 30℃. The diet of T. confusum was a mixture of regulated flour and old diet and waste from the larger stock population of T. confusum, also called frass. Regulated Flour contained whole wheat flour that was autoclaved and a 5% yeast mixture to provide a protein supplement. Frass harvested from infected populations allowed gregarine parasites to infect the diets of the new larvae. The uninfected (or “clean”) population was given 5g of regulated flour and 5 grams of clean frass (n=~600). The infected (or “dirty”) population was given 5g of regulated flour, 1.25g of clean frass, and 3.75g of dirty frass collected from a parasite-infected population (n=~600). Gregarine parasites are of the apicomplexan family and inhabit the intestines of invertebrates. While neogregarines are deadly for invertebrates, eugregarine parasites limit immune response but do not kill organisms [5]. The exact species is unknown due to naturally occurring infections from wild-caught progeny.
Sex separation and Secondary Infection. At the pupal stage, after approximately 3-4 weeks old, each group was sieved with a 710 μm sieve and separated by sex under a microscope. Virgin females have been shown to have the strongest immune response compared to mated females and males due to a stronger natural desire to survive. [6] This study used virgin females from uncontaminated lab stocks of Snave Tribolium confusum that were monitored throughout their life cycle. Before pupation, the gut wall is shed, which eliminates early-life parasites naturally. This study tested the survival of females only. The beetle pupae were sprayed with sterile Millipore H₂0 to wash the remaining flour, then a 1% Virkon (bleach) solution to disinfect any remaining parasitic infection from both the clean and dirty populations. Pupae were then given approximately 2g of flour in case of maturation.
After 3-5 days, once approximately 400 pupae had reached adulthood, 100 clean beetles were given parasite-infected diets, and 100 clean beetles were given clean diets. Conversely, 100 beetles who were initially infected with parasites were given infected diets, and 100 were given clean diets. (Fig I.) The four groups of adults were given the same diets as the original infection.
Figure 1. Roughly 1,200 T. Confusum were raised from eggs and fed diets of flour, yeast, and infected and uninfected waste from older beetle populations. The two initial infection groups were divided again, and the four smaller groups were given either the parasite-infected diet or the parasite-free diet. After two weeks of adulthood to allow for parasitic reinfection, the four groups were pierced with a Bacillus thuringiensis-covered needle (n= 64 per group) or a mock infection (n=16 per group) between the head and the thorax.
Bacterial Infection to Challenge Fitness. After fourteen days of the reinfection period, the injection protocol was executed with Bacillus thuringiensis. Using two trials, we conducted one injection protocol with a low dosage of B. thuringiensis and another with a high dosage. The bacteria were stored at -80℃ and incubated with a liquid diet of 500 mL of Millipore H₂0, 5g NaCl, 5g of Tryptone, and 2.5 grams of yeast extract. This culture was grown for 17 and then diluted and measured at an Optic Density (OD) of 600nm. We found a bacterial concentration of 3.515 x 109 Colony Forming Units/mL for the low dose, and 9.716 x 1010 CFU/mL for the high dose. To confirm colony concentration bacteria were plated with an agar solution.
Adult beetles were injected with B. thuringiensis between the head and the thorax (septic injury method [5]) using a 0.1mm minutien pin under a microscope and placed in individual wells in a 96-well plate. (n=224) Pins were dipped in this insect saline solution, then the B. thuringiensis solution. The solution is used to carry out mock infections and sterilize needles in between injections. Mock infection was carried out with 16 beetles for each parasitic infection group (n= 64) by stabbing them with only the insect saline to control for death by handling. Bacteria were injected into 64 beetles from each history group (n=256). The four groups are categorized by initial infection (gregarine positive or gregarine negative) and secondary infection (gregarine positive or gregarine negative). Survival was checked after four hours, hourly for 24 hours, then again at 48 and 72 hours. At each stage of separation and handling, a gut dissection was performed to assess the parasite prevalence within the organism population (n=10 unsexed, 10 Virkon treated, 10 clean, 10 dirty).
Statistical Analysis. Survival rates were analyzed using the Cox proportional hazard ratio for over 24 hours. Regression coefficients used were B. thuringiensisinfection status and hazard ratios were parasitic infection history. This assumes that the hazard ratio for groups with eugregarine parasites early in life, or at both points in life will be greater than one. However, groups with only late-life infection or no infection will be less than one. Groups with no B. thuringiensisinfection will also have a hazard ratio below one.
RESULTS.
Analysis of Low-Dose Bacterial Infection. After the low dose of B. thuringiensis was administered, we found an overall 7.6% mortality in all beetles given the bacterial or mock infection. The survival analysis showed no significant difference between groups with different parasitic infection histories or between bacterial infections. (pvalue = 0.168) (see Fig 2A.) No differences existed between groups, no matter their initial parasitic exposure status. The average bacterial concentration of the low dose was 3.515 x 109 CFU/mL for the injected organisms. It could be possible that the confusum beetles are better able to clear the bacterial load or better able to tolerate bacterial infection at this low infection dose. We need further experiments such as quantifying the internal bacterial load to dissect the role of infection resistance and tolerance during co-infection between parasites and bacterial infection.
Figure 2. Survival curves for T. confusum female beetles exposed to (A). gregarine parasite and a low dose of B. thuringiensis bacterial co-infection. No significant difference between hazard levels between initial infection, secondary infection, and bacterial injection. (n=288) (B). beetles exposed to the gregarine parasite and a medium dose of B. thuringiensis bacterial co-infection. In the second Trial, there was a significant difference between the Mock infection with insect saline and B. thuringiensis bacterial infection. Samples that were infected with the bacteria were more likely to die than the mock infection group. (n=320)
Analysis of Medium Dose of Bacterial Infection. The second trial with a higher dosage of B. thuringiensis resulted in a mortality of 51.11% in beetles with bacterial infection versus the 15.625% control beetles given the mock infection. Overall, bacterial infection status had a significant impact on beetle survival with and without gregarine parasites. Confusum beetles without any gregarine parasites showed marginally non-significant mortality (p= 0.06) after B. thuringiensis bacterial infection compared to their mock-infected counterparts. However, when the confusum beetles with gregarine parasites showed increased mortality compared to mock-infected beetles (p = 0.02). The mock infection groups and populations had significantly lower mortality compared to bacterial infection. However, the differences between parasitic infection histories were insignificant. There was no difference between the parasite history, but having bacterial exposure increased the likelihood of death to the organism. (see Fig II.B). The Colony Forming Unit for trial two was 9.716 x 1010 CFU/mL. We also calculated the risk or hazard ratio using the cox-proportional hazard method – that is, the rate at which the B. thuringiensis-infected beetles die compared to mock-infected beetles [5]. Here, we find that confusum beetles with gregarine parasites are approximately twice as likely to survive surviving B. thuringiensis infection when compared to beetles without gregarine parasites.
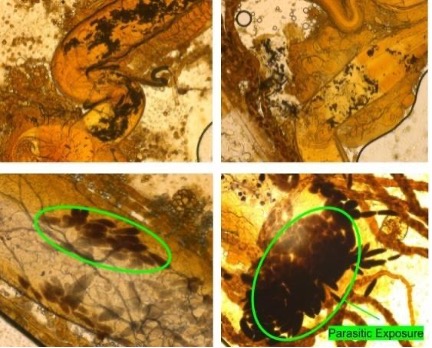
Assessing Parasitic Prevalence. Gut dissections showed that no beetles that were initially uninfected showed signs of contamination, and 10/12 samples in trial one showed high numbers of gregarine prevalence in originally infected populations. (Fig 3.) After Virkon treatment, both populations of no initial infection and initial infection showed no parasite prevalence. Reinfection of both initially and uncontaminated populations showed limited gregarine prevalence at about 2/24 samples. In trial two, there were no gregarine parasites found in sample populations that had no initial infection or in samples that were sterilized with the Virkon treatment. There were no parasites found in the second trial for samples that had never been infected, or in samples that had been treated with the sterilization treatment. There was limited parasite prevalence in both groups that had been reinfected with about 1/6 for both.
Figure 3. Gut dissection showed no gregarine parasites in populations with no initial infection (top left), or those that have been treated with 1% Virkon treatment (top right). 2/24 showed limited infection in secondary exposure, while initial infection showed 10/12 samples had sizeable gregarine prevalence (bottom left and bottom right). Gregarine parasites are dark oblong figures in the bottom images, other dark spots are intestinal material and flour.
DISCUSSION.
Conclusions from Parasites Infections Altering Fitness. In the present work we investigated the ability of confusum beetles to survive coinfection with a naturally occurring gregarine parasite and their bacterial pathogen. Here we present the evidence that female T. confusum that have been infected with gregarine parasites at any life stage have significantly lower survival rates after bacterial infection than those with no bacterial infection.
The low bacterial concentration (CFU) decreased the death rates of the model organisms. We further show that a higher dosage allowed for more significant mortality. There were negligible differences between the parasitic history groups, but a significant difference between the groups with no bacterial infection and those injected with B. thuringiensis. This shows that the model species had limited reaction to the parasites in their gut, no matter the period in their life cycle, or with the dosage of the bacteria. The sensitivity of the invertebrates’ survival due to parasitic co-infection was not altered, but a high dosage did increase mortality.
We predicted that organisms with exposure to gregarine parasites would have lower survival rates when challenged with additional B. thuringiensis infection. However, we found no change in survival between sample groups after they had been injected with bacteria. Other research found that gregarine parasites increase immune response once expelled from the larval gut of flour beetles or hurt the ability to survive once the build-up has been spent. [7]. One possibility could be that we only used adult beetles & larvae might expel gregarine at higher rates than adults [8].
Still, there is conflicting information about the effects on the immune health of gregarine parasites in numerous organisms. In a study on wild marsh-dwelling dragonflies, Erythemis simplicicolli, gregarine parasites were shown to have no connection to egg size, number, clutch size, or wing load [9] Mating that resulted in insemination in field crickets was negatively correlated with gregarine prevalence in the invertebrate [10]. Since this study only used virgin females, testing the survival of beetles after mating and both sexes may show differences between life stages of parasitic exposure. In clear and orange-winged damselflies, gregarine prevalence was negatively associated with male survival unless they could eat until satiation. [11] This study contradicts these findings in T. confusum may be the result of different immune responses, or resistance to the parasites.
Limitations and Future Directions. The nature of invertebrate model species makes determining the exact causes of death and infection prevalence difficult. This study used progeny of wild-caught beetles, which may lead to an uncontrolled parasitic prevalence or possible alternate parasitic infection. However, this is also a more realistic glimpse of natural species-parasite interaction. Future studies should consider testing the survival of bacterial infection at the larval and adult stages to determine age-based immune response. Additionally, studying the survival of both sexes and organisms that are virgins and non-virgins could dive into reproductive health with co-infection.
In conclusion, this study found that gregarine parasite history could not predict the mortality of T. confusum when infected with B. thuringiensis. The significance between groups uninfected with parasites and those infected shows that B. thuringiensis was a viable co-infection test but could not confirm the prediction that groups with longer gregarine infection would have lower survival rates. Future studies should aim to improve the sample size so clearer distinctions can be made between groups, and test both males and females.
ACKNOWLEDGMENTS.
We would like to thank Katherine Zhong and Carly Stewart for their technical assistance. We acknowledge The School for Science and Math at Vanderbilt and Rebekah Stanton.
REFERENCES
- M.A Greischar et. al. Evolutionary consequences of feedbacks between within-host competition and disease control. Evolution, Medicine, & Public Health. 1, 30-34 (2020).
- A Assefa et al, Co-infections and Comorbidities of Multiple Parasites and Hepatitis B Virus Infections in the Lowland Area of Western Ethiopia: Implications for Integrated Approaches. Journal of Multidisciplinary Healthcare. 14, 3369-3383 (2020)
- F.H.Rovenholt, A.T. Tate, The impact of coinfection dynamics on host competition and coexistence, The American Naturalist,199(1), 91-107 (2022)
- M. D. Pointer, M.J. Gage, L.G. Spurgin, Tribolium beetles as a model system in evolution and ecology, The Genetics Society, 126. 869-883 (2020)
- I. Desportes, Treatise on zoology– anatomy, taxonomy, biology, The Gregarines: the early branching Apicomplexa, Leiden: 2 158-164 (2013)
- I. Khan, A. Prakash, D. Agashe, Immunosenescence and the availability to survive bacterial infection in the red flour beetle Tribolium castaneum, Journal of Animal Ecology, 85, 291-301 (2016)
- J.Critchlow, A.Norris, A.T.Tate, The legacy of larval infection on immunological dynamics over metamorphosis, Philosophical Transactions of the Royal Society B. 374, 20190066 (2019.)
- P. Sreeramoju, M.S.K.Prasad, V.Lakshmipathi, Complete study of life cycle of Tribolium castaneum and its weight variations in the developing stages, International Journal of Plant and Animal Environmental Sciences, 6, 95-100 (2016)
- J.L. Locklin, “Gregarine parasitism in dragonfly populations of central Texas with an assessment of fitness costs in Erythemis simplicicollis” Baylor University ProQuest Dissertations Publishing (2012)
- M. Zuk, “The effects of gregarine parasites, body size, and time of day on spermatophore production and sexual selection in field crickets” Behavioral Ecology and Sociology, 21, 65-72, (1987)
- Yoshitakatsubaki, R. Hooper, “Effects of eugregarine parasites on adult longevity in the polymorphic damselfly Mnais costalis Selys” Ecological Entomology, 29, 361-366 (2004).
Posted by John Lee on Tuesday, May 30, 2023 in May 2023.
Tags: Bacillus thuringiensis infection, co-infection, parasitic exposure, Tribolium confusum