Quantifying Young’s Modulus of Gelatin Substrates for in vitro BBB Models
ABSTRACT
The Blood-Brain Barrier (BBB) is a highly selective, semi-permeable membrane that separates the circulatory system from the central nervous system. In order to better understand and predict the selectivity of the BBB, many in vitro models have been created. This research focuses on the incorporation of a gelatin substrate with tunable stiffness to improve existing in vitro models. Gels with varying concentrations of collagen were allowed to solidify inside of a Polydimethylsiloxane (PDMS) mold, then unmolded. In order to find the Young’s Modulus, a measure of stiffness, of the gels, a compressive stress test was performed using an Instron machine When plotted together, concentration and stiffness were found to have a linear relationship (R2 =.989), allowing for interpolation of data, furthering the development and modification of models without having to constantly find stiffness. This study serves as a proof-of-concept for a porcine gelatin substrate as a new in vitro model of the BBB.
INTRODUCTION.
The brain is the most complex part of the human body, allowing one to experience thought, action, and emotion, as well as serving as a hub for the Central Nervous System (CNS). As a result of its complexity and importance, the body has taken many measures to keep the brain safe. One of these defenses is the Blood-Brain Barrier (BBB), a semi-permeable, very selective membrane that separates the CNS from the circulatory system[1,2]. The BBB prevents foreign agents from traveling through the bloodstream and entering the brain, but it also affects the ability of some drugs to reach their targets.
As a result of the complexity of the BBB, many scientists have taken to developing their own models. However, even with the creation of these models, problems accurately representing the BBB have still arisen as a result of many neurological diseases (i.e. Alzheimer’s, Parkinson’s) [3,4]. These diseases alter the properties of the BBB, rendering previous models null and preventing scientists from testing the ability of certain drugs used to treat the diseases to permeate through the barrier.
In order to best predict how the BBB will react and ensure successful passage of medicine to combat neurological disorders, more representative in vitro models must be constructed [3,4]. However, as ethical pressure surrounding the use of fetal cells to form the model rises, construction becomes increasingly difficult. In order to turn away from the ethical pressure, researchers incorporated cells gathered posthumously into their in vitro models, but found that the barriers created did not have the strength and permeability found in the in vivo BBB [5]. To remediate this, many researchers have turned to induced pluripotent stem cells (iPSCs), a type of stem cell that can be generated directly from adult cells [5,6]. Once generated, iPSCs can be differentiated into many different cell types, providing readily available human endothelial cells. These endothelial cells, known as brain microvascular endothelial cells (BMECs), can be incorporated into in vitro models.
Since the discovery of the iPSC generation protocol, many different methods have been explored to create successful in vitro BBB models. Different environments have been looked at, with iPSCs being cultured in the presence of neural progenitor cells and other cells found in the neural region [6] or on different substrates [7,8]. Several differentiation processes from iPSCs to BMECs have been explored [2,5]. The empirical fashion of the research surrounding the BBB allows for almost continuous improvement of the in vitro replications of cell types found in the BBB. In order to validate research and ensure the formation of a strong barrier, cells are stained for many different tight junction proteins including Claudin-5, Occludin, PECAM-1, and VE-cadherin. In addition, trans-endothelial electrical resistance (TEER) values are measured. These values measure barrier strength, with higher TEER correlating with a stronger barrier. The presence of tight junction proteins coupled with TEER values similar to those found in vivo provide evidence that the cells are mimicking those found in vivo.
This previous research focuses on the culture and differentiation of cells that attach directly to the bottom of well plates coated with collagen and fibronectin or matrigel. While both of these substrates provide important extra-cellular matrix (ECM) proteins that give cells information, they do not replicate the stiffness of the tissue found in vivo. However, it is hypothesized that using a porcine gelatin substrate with tunable stiffness would allow TEER values to be increased due to stronger intercellular connections, which improves the strength of the BBB model.
The present work outlines the creation of a two-dimensional porcine gelatin with tunable stiffness for incorporation into in vitro models of the blood-brain barrier. Results from mechanical testing of the Young’s Modulus (stiffness) of gels is reported and correlated with the concentration of the gelatin in each model. In addition, this work evaluates the morphology of the cells that are cultured on gels with variable gelatin concentration based on their similarity to in vivo BBB cell types. This is an important first step in validating a new in vitro BBB model system.
MATERIALS AND METHODS.
PDMS Preparation.
Polydimethylsiloxane (PDMS) was prepared in order to provide a mold in which gelatin (Sigma G1890) could congeal. PDMS was poured into 50mL conical tubes along with a curing agent (1:10 ratio of curing agent to PDMS). The mixture of PDMS and curing agent was mixed vigorously until confluent. Then, 50mL conical tubes were placed in a centrifuge for 10 minutes at 1000 rpm to get rid of excess bubbles that had formed as a result of the mixing. PDMS was pipetted into paraffin molds (Fisher Scientific #22-19) around a small portion of a steel bar that was placed in the center of the mold. The PDMS-filled molds were placed on the shaker at 200 rpm until all bubbles had risen to the surface. Bubbles were removed with a micropipette. Molds were then placed into an oven at 70 C ° and allowed to cure for an hour before being taken out and carefully removed from both the plastic molds and steel rods
Sterilization of PDMS Molds.
In order to create a clean environment for gelatin formation and mechanical testing, molds and slide were sterilized. PDMS molds and glass slides (Fisher Scientific #12-544-1 25x75mm) were gathered. Three molds were gathered for every one slide. Slides were rinsed with methanol before being placed in a sonicating acetone bath for 20 seconds. The slides were then dried with N2 gas. Once dry, PDMS and glass slides were plasma cleaned. After quick removal, the plasma-treated sides of both the PDMS and glass slides were pushed onto one another, forming a bond between the base of the mold and the slide. Once the molds were securely attached to the slides, they were autoclaved on the fast cycle.
Gelatin Preparation.
Gelatin samples with varying concentrations were prepared in order to be tested on an Instron Machine. The gelatin preparation protocol was kept consistent throughout the course of the study in order to ensure easily replicated results. A 12.5% gelatin stock solution was created by adding 25g of Gelatin (Sigma G1890) to 200mL of Deionized (DI) water. This solution was placed on a hot plate at 130 degrees Celsius and 150 rpm until the gelatin powder had fully dissolved into the DI water. 10mL of DI water was added to 1g microbial transglutaminase (mTG) and placed in a bead bath and vortexed until mTG was completely dissolved. Differing amounts of mTG, gelatin stock solution, and ultrapure water were combined in 15mL conical tubes to form specific gelatin concentrations.
For mechanical testing, 2mL of each concentration was pipetted into the PDMS molds. Once filled with gelatin, the molds were incubated overnight. Gels were carved out of PDMS molds using a scoopula. Gels were placed into 50mL conical tubes filled with 30mLs of Phosphate Buffering Solution (PBS) and kept at 37 degrees Celsius until the mechanical testing took place.
For cell cultures, glass slides (Fisher Scientific #12-544-1 25x75mm) were coated with RainX. Microscope glass covers (Fisherbrand #021715-9) were plasma cleaned. 130µm of gelatin was pipetted onto the 25x75mm slide and a microscope cover was placed on top with the activated side facing down. Glass sides were placed in an incubator overnight.
Mechanical Testing.
Mechanical testing was preformed to find the Young’s Modulus of the gels. Gels were mechanically tested using an Instron Machine. Compressive stress tests at the rate of 1mm/minute were performed on the gels. After testing, extension and load values were converted to deformation (∆ height/ original height) and stress (force/area). Deformation was plotted against stress, and a portion of the graph where the R2 value 0.995 was used to find the Young’s Modulus (Stiffness).
Cell Culture.
In order to test the cell viability on the gels, iPSC-derived astrocytes were seeded onto the 7.5% gelatin hydrogels and cultured in growth medium at 37⁰C and 5% CO₂. After seven days of culture, astrocytes were fixed using paraformaldehyde and stained for glial fibrillary acidic protein (GFAP), a cytoskeletal marker of astrocytes, and the nuclear stain DAPI.
RESULTS.
Linear Relationship between Gelatin Concentration and Stiffness.
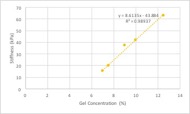
In order to ensure that slope was measured accurately, the linear portion of the graph (where R2 ≥ .995) was divided into five plots. The slopes of each individual plot were averaged together, providing an accurate and complete representation of the entire graph. This process was repeated for each of the gelatin concentrations. As shown in Figure 1b, stiffness values increased as concentration increased. When plotted together, concentration and stiffness were found to have a linear relationship (R2 =.989).
Figure 1. When plotted against one another, concentration (% gelatin) and stiffness (kilopascals) were found to have a linear relationship (R2=.989).
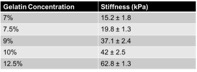
Table 1. The Young’s Modulus of 7%, 7.5%, 9%, 10%, and 12% gelatin substrates was calculated using Instron compressive stress tests. Each individual gelatin concentration was run in triplicate in order to eliminate any discrepancy. Error was found by calculating the standard deviation of the stiffness values between the three gels.
2D Gelatin Substrates Allow for Viable Cell Growth.
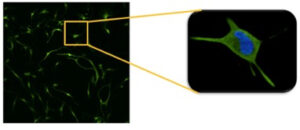
To determine whether the gelatin can be used as a cell culture substrate, astrocytes were cultured for 5 days on a 2D 7.5% Gelatin substrate. After cell culture, astrocytes were stained with DAPI and glial fibrillary acidic protein (GFAP), a cytoskeletal marker specific to astrocytes. Cells were imaged using a confocal microscope. As shown in Figure 2, astrocytes were able to attach to the gelatin substrate and display expected morphologies.
Figure 2. Mouse Astrocytes stained with GFAP (green) and DAPI (blue) imaged at 10X magnification and at 40X magnification. Astrocytes were cultured on a 7.5% gelatin substrate.
DISCUSSION.
In vitro BBB models are extremely useful. They can be beneficial in testing the ability of a treatment or drug to permeate the barrier and in examining the effect that a disease has on functionality and structure [3,4]. Models are constantly improved in order to provide a more accurate representation of the in vivo state of the BBB.
One characteristic of these models that can be modified is the substrate used for cell culture. Recently, collagen and fibronectin layers on top of well plates have been explored [7,8]. Gelatin contains tunable stiffness properties which can be changed in order to mimic different factors. Both are very viable substrate ligands as they allow cells to seed, grow, and reproduce successfully, much like they would in vivo. However, these substrates do not provide mechanical properties that are specific to tissue stiffness found around the BBB. Therefore, for this project, a porcine gelatin substrate was chosen. This substrate was coated with ECM proteins to allow the cells to culture and grow environments. The stiffness, also known as Young’s Modulus, and concentration of the gels was found to have a linear relationship (R2= .989) (Figure 1a). This relationship allows for extrapolation and interpolation of stiffness values along the curve, reducing the need for stress testing. The ease with which one is able to calculate stiffness values based on concentration is beneficial while constructing in vitro models to model certain conditions (ex. old age, Alzheimer’s, Parkinson’s).
Going forward, varying concentrations of gelatin will be used as a substrate for cell culture to see if stiffness alone can affect cell morphology [6]. Cell morphology and the ability of the cells to be seeded onto the substrate will be evaluated using DAPI and GFAP stains imaged using a confocal microscope. Along with this, a variety of cell types that make up the BBB will be seeded onto gels to see if the incorporation of the substrate improves the strength and accuracy of the model. Once seeded onto the gels, Brain Microvascular Endothelial Cells (BMECs) will be stained for tight junction proteins Claudin-5, Occludin, PECAM-1, and VE-cadherin. The presence and localization of the tight junction proteins within the BMEC monolayer provides validation to the strength of the model. Ideally, if the substrate produces cells with high levels of tight junction proteins and high TEER values, then a variety of studies could be performed using the model.
The use of a porcine gelatin substrate in the creation of an in vitro BBB model allowed for an increased variation in stiffness, providing a more accurate representation of the in vivo state. This model allows for high cell viability and fosters the reproduction and adhesion of both astrocytes and BMECs. The substrate stiffness is able to be routinely produced (see “Methods”), increasing the ability of researchers to test many levels of stiffness with high throughput. As a whole, this model contributes to the improvement of the in vitro BBB model through its incorporation of a cell-compatible substrate with tunable stiffness. It will be helpful in determining the efficacy of drugs used to treat ailments inside the brain.
ACKNOWLEDGMENTS.
I would like to thank Dr. Eeds, Dr. Lippmann, Allison Bosworth, the Bellan lab, and the SSMV class of 2018 for their continued support throughout the course of my research.
REFERENCES.
- W. A. Banks, From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
- E. S. Lippmann et al., Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 30, 783–791 (2012).
- G. A. Rosenberg, Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 32, 1139–1151 (2012).
- S. R. Caliari et al., Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci. Rep. 6, 21387 (2016).
- E. K. Hollmann et al., Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids Barriers CNS. 14, 9 (2017).
- E. S. Lippmann, A. Al-Ahmad, S. P. Palecek, E. V Shusta, Modeling the blood–brain barrier using stem cell sources. Fluids Barriers CNS. 10, 2 (2013).
- B. Trappmann et al., Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 11, 642–649 (2012).
- R. S. Stowers, S. C. Allen, L. J. Suggs, Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. U. S. A. 112, 1953–1958 (2015).
Posted by John Lee on Tuesday, December 22, 2020 in May 2018.
Tags: Blood Brain Barrier, in vitro, Young’s Modulus