Investigating the Differences in Brewing Quality Between Wild and Domestic Yeasts
ABSTRACT
It is hard to determine what caused the vast evolutionary diversity seen across both domestic brewhouse yeasts and their wild counterparts. However, comparing the variable qualities of commercial and wild yeasts can help elucidate some yeasts’ suitability in industrial brewing. Thus, this study sought to examine the differences between wild and domestic yeasts, analyzing the product outcome of three brewing variables: growth rate, pH levels, and alcohol content. Further, it additionally compared how specific wild and/or hybrid yeasts perform relative to the average domestic brewing yeast. Three experimental “wild” yeasts were selected—the S. cerevisiae var. boulardii, S. cerevisiae var. uvarum, and B. bruxellensis—with the domestic S. cerevisiae acting as the industry standard control. Each of the four yeasts fermented for two weeks following inoculation, during which the cell density and pH were measured. Following the fermentation cycle, alcohol content (as determined by calculating ABV and apparent attenuation) was additionally determined. Very little difference was found in final pH and alcohol content for all four yeasts. However, through statistical analysis and visual analysis, it was determined that the uvarum strain was the most efficient of the three wild yeasts; the boulardii was, conclusively, the least attractive and efficient.
INTRODUCTION.
Origins of Domestic Fermentation.
The interwoven relationships between humans and microbes span the development of human civilization. Yeasts and their fermentation properties have greatly benefited and have become essential to society’s continuous existence. The earliest historical fermentation was a wild endeavor, producing alcohol by spontaneous contamination as opposed to intentional measures [1]. The first emergence of the techniques that have evolved into the commercial practices seen today emerged over 9000 years ago, with early brewers inoculating new wort (the beer prior to fermentation) with a previous batch’s yeast. The impurities notwithstanding, repeated use of the same batch of yeast resulted in the gradual evolution of yeasts most effective for industrial brewing [2]. The outcome is a product with qualities unique to a particular region and favorable to a commercial audience.
Wild vs. Domestic Yeast.
While the classification of wild and domesticated yeasts is broad, the general consensus throughout the brewing industry deems any yeast commonly used in a commercial practice has, at one point or another, undergone domestication, defined here as the selection and breeding of wild species that yield variants best suited to a specific, predominantly commercial environment [3]. Domestic yeasts often display genomic decay or instability, gene duplications or mutations, chromosomal disarrangement and polyploidy, and possess phenotypes suggestive of artificial selection. These yeasts rarely thrive outside of domestic industries [4]. The most common of these yeasts is the Saccharomyces cerevisiae.
In contrast, wild yeasts have little to no prior public notability. Many are seen as contaminants and/or yield undesirable traits to a particular product, such as phenolic off-flavors, poor matorise use, unwanted acidity, and an overall spoilage of the product [5]. In summary, despite a large number of yeasts falling beyond such categorization, the two can be simply defined as such; domestic strains are found and used on a large scale in commercial, industrial, and scientific practices, and wild strains are not.
Common Brewing Variables.
The selection of brewing yeasts in commercial industries is determined by many factors, like the ability to yield attractive tastes, aromas, and textures influenced a strain’s commercial career. Manipulation and selection to gain such traits has caused a genetic and evolutionary distance of domestic strains from their wild counterparts, through both human intervention and natural development. The domestication of Saccharomyces, the most common industrial strain, epitomizes this phenomenon.
Though the smell and taste of a product heavily influences potential commercial success, such variables are generally subjective [6]. To best quantitatively represent a yeast’s attractiveness, standard variables such as alcohol content, growth rate, and pH are considered holistically with qualitative phenotypic features. On a larger scale, such variables can provide a better insight into the genetic and phenotypic characteristics that reflect a yeast’s evolutionary history, adjacency to domestic strains, and potential commercial use. Additionally, examining contrasts in a yeast’s brewing quality can provide insight into the development of yeast domestication and diversity beyond just brewing [7]. Thus, in order to explore these areas, this study sought to investigate the industrial contrasts between wild and domestic yeasts through common, qualitative brewing variables—pH level, growth, and ethanol production.
MATERIALS AND METHODS.
Experimental Overview.
The differences between four yeasts were studied over the course of two weeks in the on-site lab in a local craft brewery. Out of these treatments, the house yeast, S. cerevisiae, acted as a control. The other three experimental “wild” strains were selected from a local collection; these yeasts included two S. cerevisiae hybrids, and one of the Brettanomyces genus, a close relative to Saccharomyces. The experimental yeasts grew to a substantial amount for about a month prior to data collection. Each strain was then divided into three replicates per treatment and inoculated into 1500 mL of standard brewery wort made of a blend of malts in the style of a typical German Gerst. All replicants and yeast treatments were kept in similar environments at room temperature (~20℃). The control yeast was collected from the brewery, and because each strain had a varying cell density, the mass of the yeast introduced to the wort was different with each treatment. The yeasts’ cell density and pH levels were measured daily for two weeks and averaged amongst the three replicants for each strain.
Yeast Treatments.
With the control set as the standard house yeast, S. cerevisiae, the three experimental strains were Brettanomyces bruxellensis, Saccharomyces cerevisiae var. boulardii, and Saccharomyces cerevisiae var. uvarum.
These yeasts are not widely available in a brewing environment, but they all have a history in domestic brewing (Table 1). For instance, B. bruxellensis and uvarum strains both have a history of use in brewing; the boulardii is a pharmaceutical probiotic, sharing >99% of its genomic content with S. cerevisiae [8]. Despite this, the three yeasts fail to meet the general requirements to be considered domestic, and are still commonly considered to be contaminants in industrial brewing.
Table 1. Estimated Fermentation Data.
| Origin | Estimated Attenuation | Estimated Final pH | Temperature | |
| B. bruxellensis | Belgium | 80-90% | 3.9-4.3 | 20-22℃ |
| S. var. uvarum | Norway [9] | 76-86% | 4.3-4.6 | 24-37℃ |
| S. var. boulardii | Tropics | 70-80% | 4.4-4.6 | 20-22℃ |
| S. cerevisiae | Global | 75-80% | 4.2-4.3 | 30-35℃ |
The data in Table 1 was collected from previous brews of each yeast and from the yeast supplier. This information is commonly what is advertised to breweries and helps influence whether or not a particular yeast is selected. The origin of the yeast often correlates to what style of beer is desired (lambic, English ale, etc.), whereas the temperature relates to the temperature at which the yeast ferments best. The estimated attenuation and final pH predict the general range yeasts commonly produce in their optimum conditions.
pH Level.
Measurements regarding pH levels collected with a standard pH meter were collected at the same time as cell counts were conducted. Due to the wort used, all treatments began at a pH of 5.56.
Growth Rate.
Each of the twelve replicates was recorded daily and averaged between the three per yeast treatment. To perform cell counting, one milliliter of yeast+wort was collected and subsequently diluted per the industry standard for cell counting using a hemocytometer. The following equation depicts the calculations for determining the cell count for each replicate.
\[\frac{25(Average\ cell\ count)}{0.0001mL}\times\ (Dilution\ Ratio)\ \times\ (Total\ Growler\ Volume)\tag{Eq. 1}\]
Alcohol By Volume & Apparent Attenuation.
Two measurements were calculated in regard to the production of ethanol in each replicant: alcohol by volume (ABV) and apparent attenuation (percentage of sugars converted to ethanol and CO2). In order to find these, the initial specific gravity (IG)—the density of the initial wort+yeast divided by the density of water—was calculated against the final specific gravity (FG), which was measured following the two-week fermentation cycle. The IG for all four treatments was 12°P, as this refers to the wort used. Using an online calculator and the equation below, the ABV and apparent attenuation data was calculated.
\[(IG-FG)\times100({\frac{1.05}{0.08})=(IG-FG)\times131.25)}\tag{Eq. 2}\]
RESULTS.
Average pH Levels.
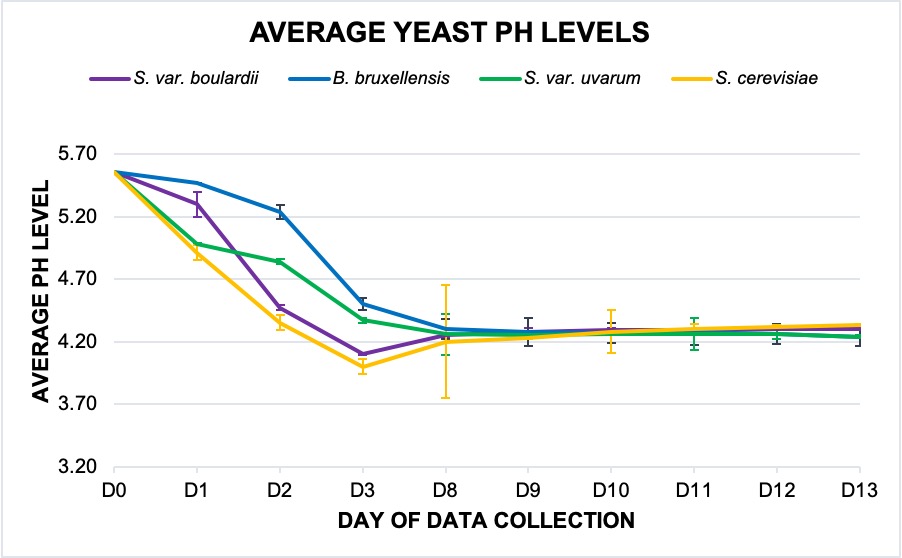
Following inoculation, all four treatments became more acidic, with the S. cerevisiae control yielding the most dramatic decrease in pH compared to the other strains (Fig. 1). Initially, the S. cerevisiae and S. cerevisiae var. uvarum had similar pH levels; however, by the third day, the uvarum hybrid diverged and gained similarities with the bruxellensis. By the end of the investigation, the cerevisiae and the boulardii probiotic ended with an average pH of 4.3, with the uvarum and bruxellensis strains both at approximately 4.24.
Figure 1. Violet: S. cerevisiae var. boulardii; blue: B. bruxellensis; green: S. cerevisiae var. uvarum; yellow: S. cerevisiae. The averages for each yeast are graphed and depict the trend in pH for each strain for a two-week period. The data from for D4 to D7 that has been excluded, which is seen in the jump from D3 to D8. This was due to an instance of acidic contamination that affected the validity of measurements collected for S. cerevisiae and S. var. uvarum. S. cerevisiae data was affected all four days, and the error only occurred in the S. var. uvarum on D7. The two other strains were not affected. Data was measured using a standard pH meter.
A Two-Factor ANOVA without replication conducted on the average daily pH measurements determined there was no present interaction between data points and yeast sample. However, evaluation of the averaged data yielded a significance between the daily measurements (p-value: 1.6×10-10); a significance between the four yeast treatments was subsequently determined (p-value: 0.00081). Further analysis found that this significance present in both daily measurements (p-value: 0.00002) and between yeast treatments (p-value: 0.033) was only present within the first four days of data collection. No significant differences were observed following the sixth day (p-value: 0.263; 0.773).
Yeast Growth Rate.
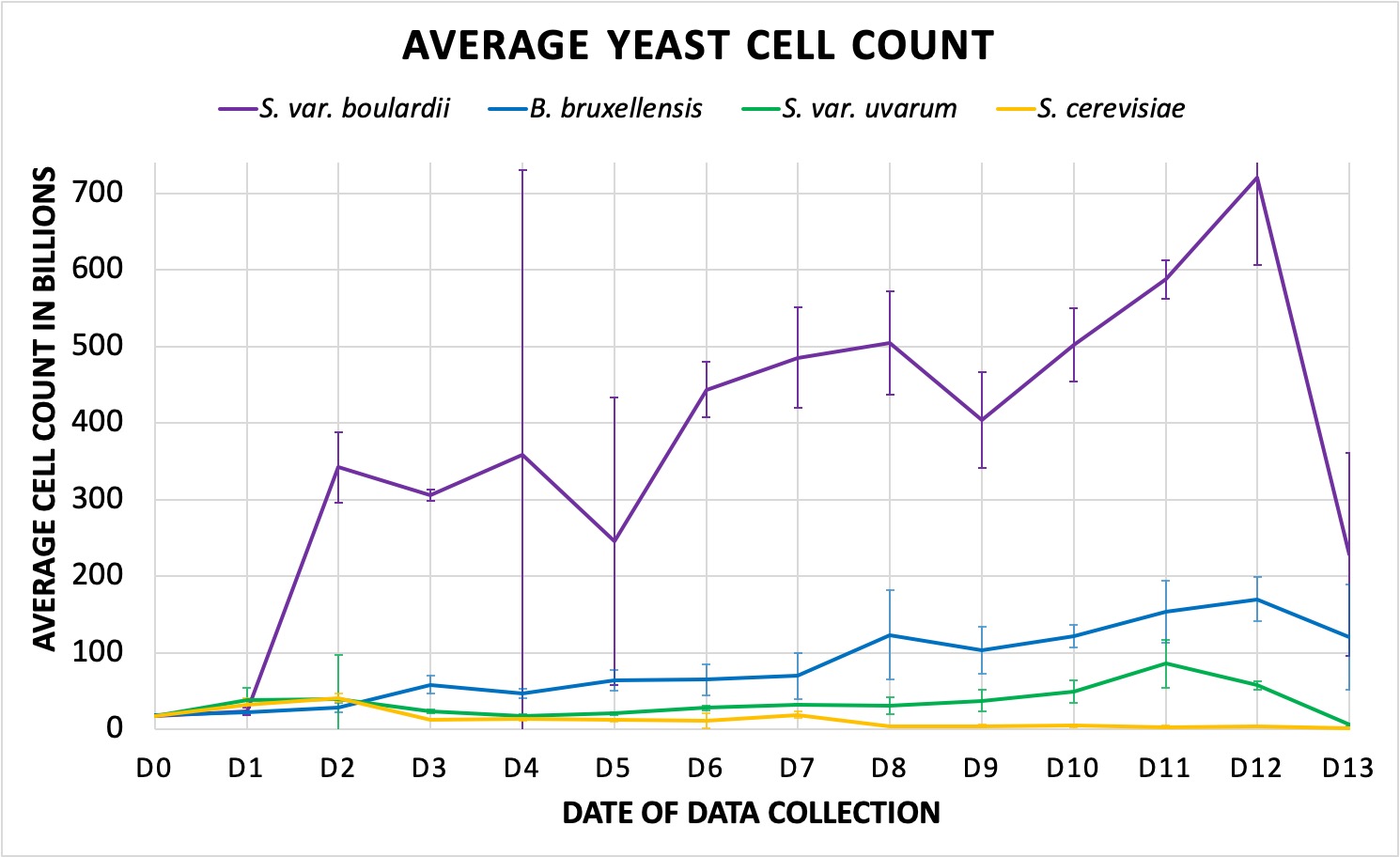
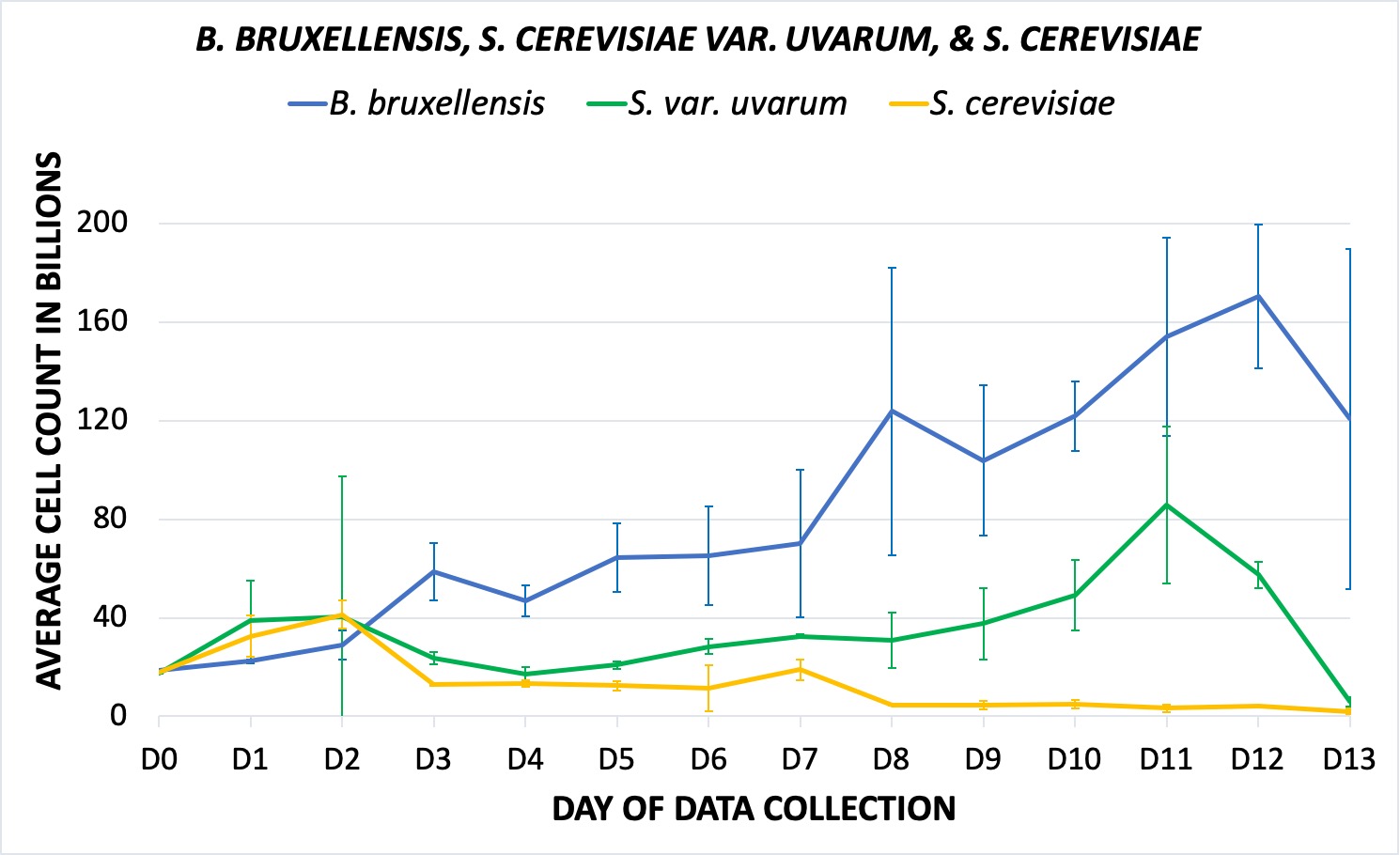
The S. cerevisiae control had the most standard growth rate for a domestic yeast, and thus provided the lowest total cell count, which suggests a short fermentation cycle. The house yeast and its uvarum hybrid, once again, saw similarities in initial growth (Fig. 2). Furthermore, though total cell growth for the bruxellensis was determined to be greater than the uvarum and cerevisiae samples, it had a considerable delay compared to the other strains; significant exponential growth did not appear until a day or two following the other yeasts, and it did not reach its peak density until final days of data collection. Still, however, by the final days of data collection, it and S. var. uvarum saw more similarities than the uvarum and the domestic control.
|
|
Figure 2. Shows average cell count with standard deviation (y-axis) in the millions over the course of two weeks for the S. var. boulardii (violet), B. bruxellensis (blue), S. var. uvarum (green), and S. cerevisiae (yellow, control). Data graphed in the billions (x109), is made up of the averaged of the three growler replicants for each yeast treatment. The slope reflects the overall trend in cell density and growth. A. Visual representation of all four yeasts, including S. var. boulardii. B. Excluding the boulardii hybrid, visual growth trend of B. bruxellensis, S. var. uvarum, and S. cerevisiae (same colors delineation as A).
The S. cerevisiae var. boulardii achieved the most dramatic growth in cell density, saw the largest variation in cell density calculations per day, and yielded the most fluctuations (Fig. 2a). There were three points of increase following the beginning of its LOG stage, a trend frequently seen in bacterial cultures. An ANOVA on the averaged growth data determined that, like pH, the null hypothesis was rejected for the four yeasts, proving that there was a significance between them (p-value: 2.0×10-12). There was no significance, however, in growth (p-value: 0.111); visual representations of this contradict this finding, though the lack of statistical difference can be attributed to insignificant variation in day to day growth or between the first and final days of measurements, as they were notably within a similar range.
Alcohol Content.
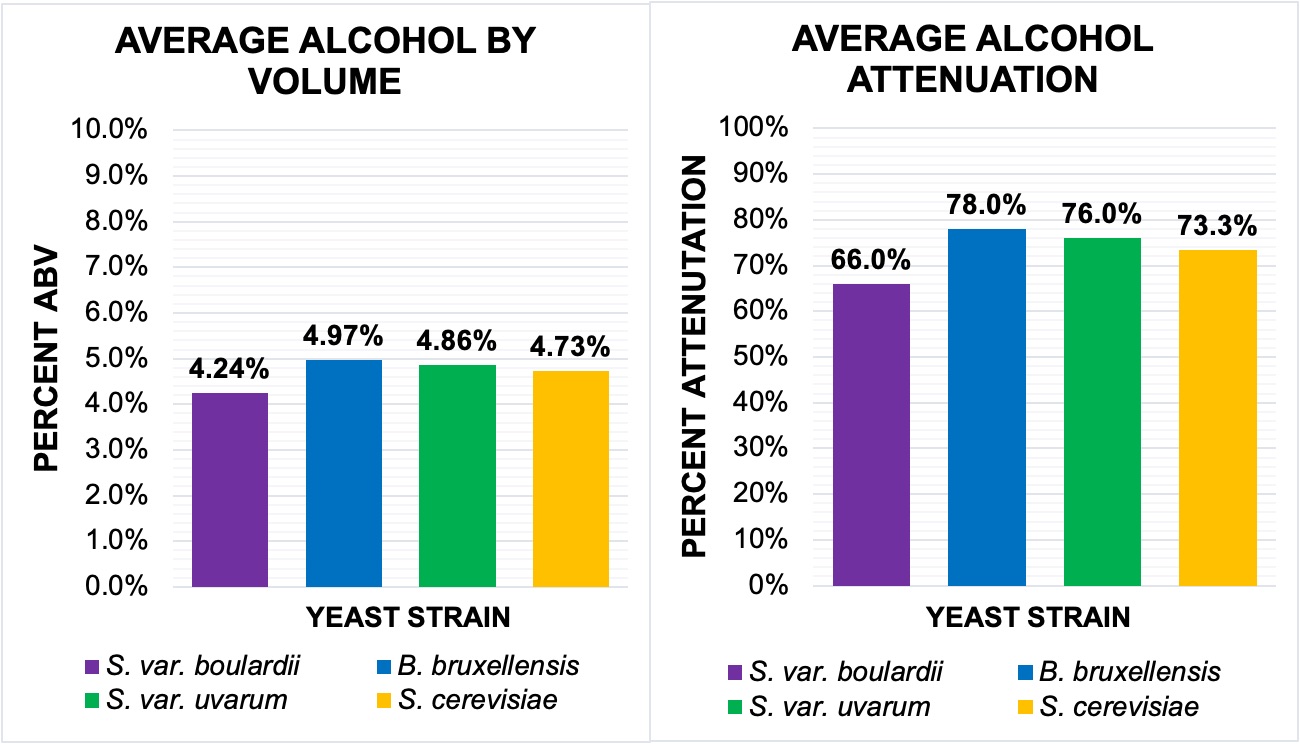
Measuring the alcohol percentage, or, more specifically, the alcohol by volume and apparent attenuation can provide important insight into the quality of a yeast’s fermentation. While the S. cerevisiae var. boulardii may have the most drastic growth out of the four yeast treatments, it produced the lowest alcohol content at 66% attenuation with an ABV of 4.24% (Fig. 3). The next lowest alcohol content was the S. cerevisiae control (73.3%, 4.73%); though the cerevisiae and its uvarum hybrid shared similar results in growth rate and pH, the S. cerevisiae var. uvarum’s calculations were conclusively closer to the data measured for B. bruxellensis. The bruxellensis yeast (78%, 4.97%) had the highest concentration. A subsequent ANOVA determined there was no significant difference between the four treatments (p-value: 0.423). From the data, this is additionally evident, as all attenuation rates were within a 12% margin, and the ABV ranged from 4.24% to 4.33%.
Figure 3. The higher the attenuation, the more alcohol has been produced. B. bruxellensis was found to have yielded the highest alcohol content, and the S. var. boulardii had the lowest; however, there was no significant difference between the four treatments. A. Average Alcohol By Volume. Same color delineations as Figures 1 and 2 (see figure key). Displays the average percent of alcohol in 1 mL of wort+yeast. B. Average Alcohol Attenuation. Same color delineations. Refers to the average percentage of sugars converted to ethanol and CO2. Data between ABV and attenuation correlates, though the two are set on different scales.
DISCUSSION.
Yeast Strain Impact on pH.
The pH a particular yeast yields is a key indicator of the commercial profitability of the overall brew; a pH that is too high can lead to bitter off flavors, poor fermentation, and a greater risk of spoilage and bacterial contamination [10]. The pH for S. cerevisiae, for the brewery house yeast used as a control, ranges, on average, from 4.2 to 4.3. According to the trend in average pH, following the yeasts’ entrance into their respective LOG stages, a rise in acidity continued for about ten days before it steadied out to the predicted level for all treatments. There was no sample that experienced any pH that significantly exceeded the predicted domestic range. A Two-Factor ANOVA did determine that significant change occurred within the first four days, but by the ninth, there was no statistical difference between the four yeasts or the daily measurements, corroborated by the trend seen through the averaged pH levels during this period.
Change in Cell Density During Fermentation.
Notably, S. cerevisiae var. boulardii experienced the largest growth over the fermentation period. The pattern of cell proliferation was largely inconsistent, characterized by a three-tiered fermentation trend. This is particularly interesting, as such trends are common in typical bacterial laboratory cultures. As such, the boulardii’s common use as a probiotic rather than a brewing yeast could be responsible for its growth patterns, though further genetic analysis is needed to fully determine and understand the cause of this unusual fermentation pattern. As for the cell density of the other yeasts, S. cerevisiae and S. cerevisiae var. uvarum began with similar growth; by the end, however, the latter was more similar to the Brettanomyces strain, affirming that the domestic control saw the quickest and most efficient fermentation in regard to typical brewhouse demands and had the lowest continuous density in cells during the two weeks of fermentation. On the other hand, the slowest of the four was determined to be B. bruxellensis; historically, it has been known to yield longer fermentation cycles, and though it does develop at a slower rate, it is relatively efficient in terms of the types of beers it tends to produce.
Interpretations: Alcohol Content & Production.
Calculating alcohol content and ethanol production relies on the initial conditions of the wort each yeast is launched in, though it is less influenced by external factors such as pH level. The inoculation of domestic S. cerevisiae into the wort used in this investigation results in, on average, an attenuation of 73-74% and an ABV at 4.90%; this corroborates the alcohol data found for the control (73%, 4.73%). Furthermore, the boulardii and bruxellensis both are reported to perform best at 20-22℃. Therefore, the high attenuation reflected by the bruxellensis strain and the low alcohol content seen from the boulardii can be interpreted as a direct result of the yeast’s individual performance and can be used a relatively accurate portrayal of the variable differences that these two experimental “wild” yeasts produce in regards to a domestic strain. Analysis of the ABV and apparent attenuation between the four yeasts found that there was no significant variance between the concentration of ethanol present, nor in the percent success of glucose conversion during fermentation. Despite a lack of statistical significance, acknowledging the present contrasts between the strains is still noteworthy when comparing both the phenotypic and genetic characteristics of different yeasts. From an industrial perspective, the combination of this data with pH and growth rate, can help evaluate the selection of a particular strain.
Project Limitations & Future Directions.
The presence of human error and potential bias is a restriction that goes without saying. For instance, to measure growth, cells of each 12 replicants were manually counted under a hemocytometer and calculated using a standard formula. The reliance on the averages of such a small sample size is bound to yield some error in the analytical representation of the data. Additionally, the pH data for the S. cerevisiae and the uvarum hybrid saw an instance of acidic contamination. Fortunately, the issue—a mishap in the dilution process necessary for cell counting—was amended within a few days; relative to the two yeasts unaffected, the odds that this affected the pH data in any significant matter is slim, but this error is still important to note.
This is nothing, however, that cannot be prevented in future research. Replicating this study could provide further insight into the variations between the four yeasts it focused on. Additional experimentation might explore a larger sample of yeasts, both wild and domestic; studying more variables and quality-indicators is also an option. It would also be interesting to develop new variables, such as methods of exploring genetic and phenotypic variations that determine a yeasts’ domestication or potential commercial use. Strains farther removed from domestic species, especially those outside of the Saccharomyces genus, are of particular interest to study in the future. Variables regarding the wort used in inoculation, the fermentation temperatures, and other typical brewing determinants that were excluded from this study are also up for grabs. In all, examining avenues (be it brewing variables, conditions, or outcomes) that allow for further insight into the evolution of yeasts, the contrasts between wild and domestic strains, and their prospective future in the modern industry are of most importance.
ACKNOWLEDGMENTS.
Special thanks to Linus Hall and Yazoo Brewing Company, to Dr. Angela Eeds, Dr. Kathrine Friedman, Katrina Ngo, and the SSMV Class of 2022 for all their guidance and support.
REFERENCES
- J. Steensels, K. J. Verstrepen. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 68, 61-80 (2014).
- J. E. Pérez-Ortı́n et al. Molecular Characterization of a Chromosomal Rearrangement Involved in the Adaptive Evolution of Yeast Strains. Genome Res. 12, 1533–1539 (2002).
- B. Gallone et al. Domestication and divergence of Saccharomyces cerevisiae in beer yeasts. Cell. 166, 1397-1410 (2016).
- C. A. Driscoll et al. From wild animals to domestic pets, an evolutionary view of domestication. Proc. Natl. Acad. Sci. 106 (supp. 1), 9971–9978 (2009).
- Z. Mu, X. Yang, H. Yuan. Detection and identification of wild yeast in Koumiss. Food Microbiol. 31, 301–308 (2012).
- J. De Keersmaecker. The mystery of Lambic beer. Sci. Am. 275, 74–80 (1996).
- C. D. Powell, A. N. Diacetis. Long term serial re-pitching and the genetic and phenotypic stability of brewer’s yeast. J. Inst. Brew. 113, 67–74 (2007).
- I. Khatri et al. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 7, 371 (2017).
- K. Krogerus et al. A unique Saccharomyces cerevisiae × Saccharomyces uvarum hybrid isolated from Norwegian farmhouse beer: Characterization and reconstruction. Front. Microbiol. 9 (2018).
- C. Bible. The Principles of pH. Brew Your Own. (2007)
Posted by John Lee on Saturday, May 7, 2022 in May 2022.
Tags: alcoholic fermentation, brewing, microbiology, Yeast domestication, yeasts