In Vitro Analysis of Synergy between Adjuvants MPLA, CpG, and cGAMP for Treatment of Cancer
ABSTRACT
Immunotherapy, the treatment of diseases by inducing, enhancing, or suppressing immune responses, provides far greater potential for successful cancer treatment as opposed to traditional chemotherapy. Immunotherapy eradicates the risk of metastasis and provides more safe, efficient, and effective treatment in cancers. Using specific biological compounds, or adjuvants, activates responses in the body through specific molecular pathways. The objective of the research was to determine if synergy existed between adjuvants MPLA, CpG, and cGAMP. Multiple adjuvants result in the activation of multiple pathways simultaneously; the existence of synergy between two adjuvants indicate immunological responses are substantially higher due to an enhanced activation of multiple pathways. RAW Dual cells were dosed with one adjuvant at a set concentration and the other at doses, including the IC-50 and concentrations of twice, quadruple, half, and a fourth of the IC50. Quantiblue, Quantiluc, and Cytotoxicity assays were conducted to quantify IFN and NF-κB pathway activation and cell death. The extracted data suggest synergy is apparent between all combinations of the tested adjuvants. The resulting immunological responses of synergistic application of adjuvants, when compared to baseline application of a single adjuvant, is substantially higher. Resulting data may be used to improve current cancer treatments.
INTRODUCTION.
Although cancer is one of the most lethal diseases, its most common treatment, chemotherapy, is not optimal. Immunotherapy for treatment of cancer retains far greater potential than traditional chemotherapy [1]. Chemotherapeutic treatments consist of injecting patients with multiple cytotoxic drugs through intravenous infusion that aim to eliminate cancerous cells. This, however, encompasses numerous complications that impact the patient’s health and safety [2]. By injecting patients with drugs, cell death is not targeted, and healthy cells are killed in the process [3]. Thus, both malignant cells and normal cells proximal to the bloodstream are eradicated, which places patients at a potential risk of organ failure or death. Furthermore, chemotherapy damages the underlying bone tissue and bone marrow which provides further limitations since bone marrow produces blood cells [4]. As a result, less erythrocytes and/or hemoglobin is produced, which decreases the amounts of oxygen and nutrients circulating throughout the body. Significantly less leukocytes are available which cripples the immune system’s ability to target infections and or pathogens. Further, less platelets are produced, which limits the body’s ability to create clots, thereby increasing blood loss from injuries. Moreover, chemotherapeutic treatments only work to kill cancerous cells; however, the treatments do not eliminate the risk of tumor metastasis, only reducing risk at best. Contrarily, immunotherapy provides a safer, more efficient, and more reliable alternative to chemotherapeutics.
Immunotherapy, in broad terms, activates the immune system to prompt immune cells to recognize cancer cells as foreign objects or neoplasms and kill them [5]. Within immunotherapy, there are two branches: innate and adaptive. The innate aspect utilizes the capabilities of the innate immune system to initiate targeted immunological responses to destroy malignant cells. The adaptive, however, uses immune cells – such as T cells – that eliminate pathogens and diseases and evince the creation of antigens. Within this study, innate immunotherapy is studied. Because they resemble somatic cells, tumor cells can often circumvent detection by the immune system. Thus, by utilizing immunotherapeutics to activate the immune system, the immune system can target and eradicate tumorous cells like it would a pathogen [1]. By activating the innate immune system, tumorous microenvironments are stimulated and made more active, which means there is a greater chance that the adaptive immune system can target and kill cancerous cells [6,7]. The innate immune system, once it recognizes the cancer cells, signals dendritic cells which then present cancer-specific antigens to CD8+ T cells which can then attack cancer cells with the antigens [5,8]. In doing so, the immune system can efficiently kill cancer cells, limit off-target cell death, and prompt the adaptive immune system to create memory cells, which would be able to kill metastatic tumors [5].
In this study, the efficacy of the synergical application of three adjuvants – compounds that evoke and enhance immunological response – cGAMP, CpG, and MPLA, is examined in vitro. cGAMP, a cyclic dinucleotide, is an agonist for the STING receptor, which activates an interferon response and releases cytokines when cytosolic DNA from a virus infiltrates the cell [9-10]. CpG, an oligonucleotide, activates the TLR9 pathway, which initiates NF-κB and pro-inflammatory cytokine responses when binded to bacterial or viral DNA [10]. MPLA, a modified chemical, is an agonist for the TLR4 pathway, which evokes NF-κB and inflammatory cytokine responses when bacteria are found along the cellular membrane [10]. The adjuvants were tested synergistically within RAW Dual cells, reporter cell mouse macrophages that are engineered to secrete alkaline phosphatase and luciferase in response to immune activation, such that immunological responses can be quantified. To test for synergy, Quantiblue, which quantifies NF-κB responses, and Quantiluc, which quantifies IFN response assays were run on cells dosed with two different adjuvants, one at a fixed concentration while the other varies across a range. By examining the synergy between two adjuvants, immunological responses can be significantly furthered, thereby prompting the body to eradicate the tumor providing a more potent treatment with greater efficacy and safety than traditional chemotherapy [6,7,10].
MATERIALS AND METHODS.
To prepare for experimentation, RAW Dual cells were counted using a hemocytometer and diluted in media as necessary to obtain a 200,000 cells/mL concentration; Then, 200 μg/mL of zeocin, a selective antibiotic, is then added to cell supernatant. Next, 100 μL of the cell concentration was plated into 96-well plates such that 20,000 cells were present in each well. Plates were then left to incubate for 24 hours at 37°C. Afterwards, cells were dosed with a particular adjuvant.
Utilizing preliminary data that showed the half maximal inhibitory concentrations (IC50s), which is the concentration of an inhibitor whence response is reduced by half, of the three adjuvants. A dosing scheme was established that would ensure that cells were dosed with effective doses of each adjuvant. The IC50 of each adjuvant was doubled to create a “high” dose and quadrupled to create a “very high” dose, the inverse is done to create a “low” and “very low” concentration dose. The different doses are then used to create a dosing scheme that included many ratios of one adjuvant to the other (i.e. a very high dose of cGAMP to a very low dose of MPLA). RAW Dual cells were dosed with 100 μM to 6.25 μM of a cGAMP and 11.34 μg/mL to 0.70875 μg/mL of Monophosphoryl Lipid A; one adjuvant was applied to the cells at a single concentration while the other adjuvant was diluted serially four times by a factor of two and vice versa. The cells were dosed with the same drug concentrations in triplicates so that an average number could be used for analysis. Adjuvants were suspended in media containing the cells; media alone was analyzed to provide a base for comparison. A similar dosing was repeated for the other two synergy examinations, albeit with different adjuvants and different initial concentrations. Cells were dosed with 11.34 μg/mL to 0.70875 μg/mL of MPLA and 1.25 μM to 0.078125 μM of CpG to test for synergy. Previous data that tested the synergy of CpG and cGAMP at the aforementioned concentrations were used for comparison. After dosing, cells were left to incubate for 24 hours. Post-incubation, Quantiblue and Quantiluc assays, were conducted on cells to quantify responses.
Quantiblue is an assay that determines the amount of alkaline phosphatase, an indicator that an NF-κB immunological response occurred, that is secreted. Quantiblue is conducted by adding 180 μL of the Quantiblue reagent to 20 μL of cell supernatant into a 96 well plate. The plate is analyzed using an absorbance at 625 nm in a plate reader after 1-hour intervals, with a final reading at 4 hours. The control media – with no added adjuvants – absorbances were subtracted from the absorbance results of each group to find how much higher activation was from a base comparison. The average of the absorbance difference results was taken for each triplicate of each dose and error bars were added using standard deviations of the data.
Quantiluc is an assay that quantifies the amount of luciferase, which is an indicator that an IFN response occurred. Quantiluc is conducted by placing 20 μL of cell supernatant into a 96 well non-binding plate. The plate is inserted into the plate reader, which measured luminescence in this case, where 50 μL of Quantiluc reagent is added to the cells and the cells are analyzed immediately afterwards. The control media – with no added adjuvants – luminescences were subtracted from the luminescence results of each group to find how much higher activation was from a base comparison. The average of the luminescence difference results was taken for each triplicate of each dose and error bars were added using standard deviations of the data.
RESULTS.
The quantification of interferon and NF-KB responses were measured in response to both synergistic and single adjuvants to provide comparison. Within each graph, the respective immunological response quantification was plotted as a means of including a baseline to show the significance in the increase of response. Cells were dosed with increasing increments of an adjuvant along with a single concentration of the other adjuvant for each triplicate group. Within each comparison group, there are data that support a substantial increase in activation when two adjuvants are used synergistically as opposed to adjuvants used individually.
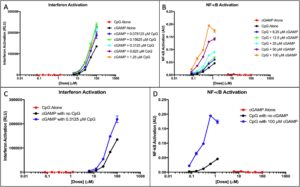
cGAMP + CpG.
A Quantiluc assay showed that interferon activation was minimal for CpG alone, which is to be expected because CpG activates NF-êB rather than IFN, and exponential for cGAMP alone (Figure 1A).
Figure 1. cGAMP and CpG IFN and NF-κB activation. (A) Interferon response of cGAMP doses with an added fixed CpG concentration was measured. The response of the adjuvants alone was measured to provide a baseline. (B) NF-κB response of CpG doses with an added fixed cGAMP concentration was measured. The NF-κB response of the adjuvants alone was measured to provide a baseline. (C) Interferon response of CpG alone and cGAMP alone at all doses was plotted. The optimal dose of the synergistic application in part A is plotted to show comparison. (D) NF-êB response of cGAMP alone and CpG alone at all doses was plotted. The optimal dose of the synergistic application in part B is plotted to show comparison.
Observation of IFN response showed that response is not dose-dependent; meaning, that at any given concentration of the added CpG, interferon response increases regardless of the specific concentration of CpG. CpG does not activate interferon, yet there is an apparent increase in response when CpG is added to the treatment.
A Quantiblue assay reveals that NF-êB activation was minimal for cGAMP, and exponential for CpG (Figure 1B). NF-KB quantities shows that activation is dose-dependent; at different doses of added cGAMP, activation varies depending on the specific concentrations. cGAMP does not activate NF-KB, yet there is a substantial increase in NF-êB response when cGAMP doses are synergistically used with CpG.
Figure 1C-D show the optimum doses shown in the synergy with all doses of both adjuvants. CpG elicits no interferon response but an exponential NF-êB response and cGAMP elicits no NF-êB response but an exponential interferon activation. When both adjuvants are used synergistically, there is a substantially higher activation in both NF-êB and interferon responses.
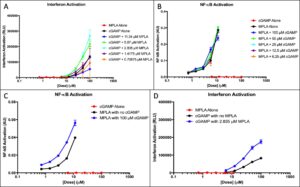
cGAMP + MPLA.
Quantiluc assays show that interferon responses for MPLA alone were dismissible, which was expected. cGAMP interferon activation was greater than what was expected and continued to increase exponentially (Figure 2A). Interferon response shows activation is dose-dependent. At specific dosages of added MPLA, cGAMP-induced interferon responses were significantly higher, nearly three times the value of the cGAMP baseline value. MPLA does not activate the interferon pathway, yet interferon activation increases when MPLA is present. Quantiblue assays reveals minimal cGAMP-induced response and a significant MPLA-induced response (Figure 2B). NF-KB quantities shows that activation is not dose-dependent; at different doses of added cGAMP, activation remains relatively uniform. When cGAMP is added to MPLA doses, the response is not significantly higher.
Figure 2. cGAMP and MPLA IFN and NF-κB activation. (A) Interferon response of cGAMP doses with added fixed MPLA concentrations was measured. The response of the adjuvants alone was measured to provide a baseline. (B)NF-κB response of MPLA doses with added fixed concentrations of cGAMP was measured. The response was measured alone to provide a baseline. (C) Interferon response of MPLA alone and cGAMP alone at all doses was plotted. The optimal dose of the synergistic application in part A is plotted to show comparison. (D) NF-êB response of cGAMP alone and MPLA alone at all doses was plotted. The optimal dose of the synergistic application in part B is plotted to show comparison.
Figure 2C-D shows the optimal doses shown in the synergy with all doses of both adjuvants. MPLA elicits no interferon response but an exponential NF-êB response and cGAMP elicits no NF-êB response but an exponential interferon activation. When both adjuvants are used synergistically, there is a substantially higher activation in both NF-êB and interferon responses.
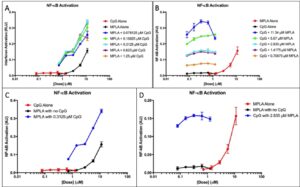
MPLA + CpG.
Both MPLA and CpG elicit an NF-êB response and neither results in IFN activation, so IFN response is not shown. Figure 3A shows that activation is dose-independent; at higher dosages of added CpG, NF-KB activation remains relatively constant but still higher than the activation of the adjuvants alone. Figure 3B shows that activation was dose-dependent. Pathway activation increased substantially as the doses of added MPLA changed.
Figure 3. CpG and MPLA IFN and NF-κB activation. (A) NF-κB response of CpG doses with added fixed MPLA concentrations was measured. The NF-κB response of the adjuvants was measured alone to provide a baseline. (B)NF-κB response of doses of MPLA with added fixed CpG concentrations was measured. The NF-κB response of the adjuvants was measured alone to provide a baseline. (C) NF-κB responses of MPLA alone and CpG alone at all doses were plotted. The optimal dose of the synergistic application in part A is plotted to show comparison D) The optimal dose of the synergistic application shown in part B is plotted to show comparison.
Figure 3C-D shows the optimal doses shown in the synergy with all doses of both adjuvants. Both MPLA and CpG elicit an exponential NF-êB response. When both adjuvants are used synergistically, there is a substantially higher activation in both NF-êB responses.
DISCUSSION.
Synergy between all combinations of adjuvants is observed. When RAW Dual cells are dosed with concentrations of cGAMP, an interferon response is observed, but no NF-κB responses occur. When CpG is added, an NF-κB response is observed, but no interferon responses occur. When MPLA is added, NF-κB response is observed, but minimal interferon response occurs. Theoretically, when cells are dosed with two of these adjuvants, neither interferon nor NF-κB activation should increase significantly. As Figure 1 shows, however, both NF-κB and interferon responses are significantly higher cGAMP and CpG are synergistically applied, which shows the existence of synergy between cGAMP and CpG. Additionally, as shown in Figure 2, both NF-κB and interferon responses are significantly higher when MPLA and cGAMP are synergistically applied, which shows the existence of synergy between cGAMP and MPLA. NF-κB results show that when CpG and MPLA are added, increased activation occurs regardless of the added dose, thereby furthering the evidence of synergy. Generally, all combinations of the three adjuvants displayed preliminary synergy, which can be enhanced by nanoparticle-mediated co-delivery of the adjuvants.
Synergy data can be used to continue adjuvant analysis to find precise ratios of added adjuvant concentration and volume to that of another adjuvant that will, when delivered in vivo, yield the greatest immunological response, the most tumor cell death, and the least off-target cell death. Ratio comparisons will allow for the development of an adjuvant treatment that will treat cancers more effectively at more efficient and lower concentrations of drugs and adjuvants. Synergy data may also be utilized in conjunction with nanoparticle-mediated co-delivery using polymersomes, which will encapsulate adjuvants to ensure more precise, safe, and efficient treatment. Aforementioned adjuvant ratios could be co-encapsulated by polymersomes to maximize efficacy in cancer research; this would ensure that precise ratios of co-encapsulated adjuvants enter all cells and initiate apoptosis or necrosis. Vaccines can be developed using synergy data and polymersome co-encapsulation data to be used in clinical trials in vivo in mice and eventually within humans to mitigate and treat cases of several different types of cancer, though more research is required to find how the efficacy of adjuvant synergy therapy differs across different cancer types.
Synergy results provided evidence that CpG and cGAMP combinations support results of a previous study [11]. However, said study does not elaborate on combinations, nor does it provide results that support the claim; the data that are revealed in this study show that the combination is viable and provides substantially greater immunological activation. Further, the data represented supports data that indicate that TLR and STING, which are activated by CpG and cGAMP, respectively, adjuvants working synergistically improves immunological responses in comparison to single adjuvant use [8]. Moreover, the synergy results provide a continuation of results reported for a study on the Tumor Micro-Environment (TME) [12]. The article indicated that cGAMP controlled tumor growth and MPLA and CpGs demonstrated TME reprogramming abilities [12]. The article only investigates the effects of the adjuvants on the TME. However, the article, nor any other article, measure the immunological response or the synergistic application of the three adjuvants.
ACKNOWLEDGMENTS.
I would like to thank Dr. John Wilson for allowing me to work in his lab, Jessalyn Baljon for her guidance and assistance throughout this project, and Dr. Angela Eeds and the SSMV for their advice and continued support.
REFERENCES
- L. Coffman, A. Sher, R. A. Seder, Vaccine adjuvants: putting innate immunity to work. Immunity. 33, 492–503 (2010).
- Nieboer, C. Buijs, S. Rodenhuis, C. Seynaeve, L. V. Beex, E. V. D. Wall, D. J. Richel, M. A. Nooij, E. E. Voest, P. Hupperets, N. H. Mulder, W. T. V. D. Graaf, E. M. Tenvergert, H. V. Tinteren, E. G. D. Vries, Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. Journal of Clinical Oncology. 23, 8296–8304 (2005).
- J. Gralla, Cannabinoids and the control of chemotherapy-induced nausea and vomiting. Marijuana and Medicine. 2001, 599–610 (2001).
- Taki, Faculty of 1000 evaluation for chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Post-publication peer review of the biomedical literature. 2013, F1000 (2013).
- S. Chen, I. Mellman, Oncology meets immunology: the cancer-immunity cycle. Immunity. 39, 1–10 (2013).
- Corrales, L. Glickman, S. M. Mcwhirter, D. B. Kanne, K. E. Sivick, E. Lemmens, J. J. Leong, K. Metchette, T. W. Dubensky, T. Gajewski, Direct activation of STING in the tumor microenvironment with synthetic cyclic dinucleotide derivatives leads to potent and systemic tumor-specific immunity. Journal for ImmunoTherapy of Cancer. 2, Suppl (2015).
- M. Whitmore, M. J. Deveer, A. Edling, R. K. Oates, B. Simons, D. Lindner, B. R. G. Williams, Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Research. 64, 5850–5860 (2004).
- Temizoz, E. Kuroda, K. Ohata, N. Jounai, K. Ozasa, K. Kobiyama, T. Aoshi, K. J. Ishii, TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. European Journal of Immunology. 45, 1159–1169 (2015).
- Cai, Y.-H. Chiu, Z. J. Chen, the cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Molecular Cell. 54, pp. 289–296, (2014).
- Li, S. Qu, X. Chen, Q. Wu, M. Shi, Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. International Journal of Molecular Sciences, 18, 404 (2017).
- Temizoz, E. Kuroda, K. J. Ishii, Vaccine adjuvants as potential cancer immunotherapeutics. International Immunology, 28, 329–338 (2016).
- Kalinski, J. E. Talmadge, “Tumor immuno-environment in cancer progression and therapy. Advances in Experimental Medicine and Biology Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy. 2017, 1–18 (2017).
Posted by John Lee on Tuesday, December 22, 2020 in May 2019.
Tags: Adjuvant, Cancer Immunotherapy, Immunological response, STING pathway