Evaluating Cell Culture Systems for Engulfment Receptor Function: Jedi-1 in BV2 and HeLa Cells
ABSTRACT
Microglia are brain-resident immune cells responsible for neuronal corpse clearance and maintaining tissue homeostasis. Recently, Jedi-1, an engulfment receptor, was shown in mice to play an important role in neuronal corpse clearance by phagocytosis. Defective engulfment receptor proteins in the nervous system have been linked to multiple disorders in development and aging, as uncleared apoptotic cells can trigger an inflammatory response and disrupt homeostasis. We sought to develop an in vitro assay to study Jedi-1 function during engulfment. BV2 cells are a murine microglia cell line but whether Jedi-1 is expressed by these cells is unknown. Preliminary data has shown that Jedi-1 expression increases in primary microglia during a microbead engulfment assay. By using a dead cell engulfment assay with BV2 cells and Jedi-1 transfected HeLa cells, this research investigates whether Jedi-1 can be induced in BV2 cells upon incubation with apoptotic neural cells during different time points of engulfment. We examined this through engulfment assays and Western Blotting. Results indicate that Jedi-1 expression is not induced in BV2 cells in the presence of apoptotic neural cells and demonstrate that Jedi-1 transfected HeLa cells are capable of engulfment. This helps us understand the role of Jedi-1 in microglia, provides a foundational assay for studying other engulfment receptors and their signaling partners, and provides a culture system to study the role of phagocytosis in neuropathologies.
INTRODUCTION.
During the development of the mammalian nervous system, about half of the neurons will undergo apoptosis, programmed cell death, as a normal pruning process. This is essential for regulating growth and homeostasis in the brain, counterbalancing the overproduction of neurons. However, dying neurons must be properly recognized and eliminated to prevent disease. Dying neurons produce “eat me” signals, usually phosphatidylserine. Microglia, the resident macrophages of the central nervous system (CNS), are responsible for recognizing these signals and clearing neuronal corpses to maintain homeostasis. Much progress has been made in understanding the regulation of neuronal cell death [1]. However, little is known about how these extensive amounts of neuronal corpses are eliminated. Throughout the development of the nervous system, microglia are involved in neurogenesis, apoptosis, synapse elimination, and the establishment of neural circuits. They display various morphologies, depending on their activation states, and take on many functions [2]. Microglia are essential in initiating repair following neuronal injury by phagocytosing dead neurons without eliciting inflammation [3].
Because microglia play an important role in maintaining synaptic pruning and maturation in the developing brain, deficits in microglial activity may cause synaptic abnormalities observed in many neurodevelopmental disorders [4]. When left uncleared, apoptotic cells undergo secondary necrosis, which triggers neurodegenerative diseases [5]. To investigate the role of microglia in apoptotic cell removal and disease, we studied the mechanisms through which microglia engulf apoptotic cells.
Present in microglia is a recently found engulfment receptor protein called Jedi-1. Previous research has found that endogenous Jedi-1 plays an important role in neuronal corpse clearance by satellite glial cells in the peripheral nervous system [6]. Here, we focus on Jedi-1 expressed in microglia in the CNS. Preliminary data from the Carter Lab has shown that loss of Jedi-1 is associated with decreased neurogenesis, decreased microglial engulfment, and increased inflammation. Prior data has shown that HeLa cells, when transfected with Jedi-1, gained the ability to engulf microbeads that mimic apoptotic cells, showing that Jedi-1 can phagocytose independently through the tyrosine kinase SYK [7]. Because of this finding, we hypothesized that Jedi-1 transfected HeLa cells would be capable of phagocytosing apoptotic neural cells, specifically SY5Y neuroblastoma cells.
Jedi-1 is similar to the triggering receptor expressed on myeloid cells-2 (TREM-2) receptor protein in many ways; it is located in the microglia, promotes phagocytosis, and reduces inflammation. As both proteins mediate engulfment through SYK, we sought to investigate whether Jedi-1 may share other TREM-2 properties. Expression of TREM-2 has been shown to increase in BV2 cells upon exposure to apoptotic cells during an engulfment assay [3]. BV2 cells are a common microglia cell line that has also shown engulfment receptor induction during engulfment, but whether Jedi-1 is expressed by these cells is unknown. Since Jedi-1 shares functional similarities to TREM-2, we questioned whether Jedi-1 expression was similarly induced in BV2 cells during the engulfment process. Preliminary lab data has shown that Jedi-1 expression in primary microglia increased during the engulfment of microbeads that simulated apoptotic cells, so we applied this rationale to BV2 cells and apoptotic SY5Y cells as a way to affirm and expand on preliminary findings. As BV2 cells are a model microglia cell line and SY5Y cells are a model for neurodegenerative disorders from a neuroblastoma cell line, it is expected that BV2 cells phagocytose apoptotic SY5Y cells. Moreover, BV2 cells have shown to be a valid substitute for primary microglia in many experimental settings, including complex cell-cell interaction studies [8]. Based on this information, we hypothesize that Jedi-1 expression in BV2 cells will increase during the engulfment of apoptotic SY5Y cells.
We aimed to discover the best model method to study the signaling pathways downstream of Jedi-1 that are altering microglia phenotype. To investigate this, we evaluated the presence and the changes of Jedi-1 levels in BV2 cells during engulfment. We simultaneously evaluated the ability of Jedi-1 transfected HeLa cells to engulf and whether Jedi-1 levels changed during engulfment.
MATERIALS AND METHODS.
- Assessing Jedi-1 Expression in BV2 Cells
1.1 Cell Culture and Transfection. BV2 cells were grown in a Mixed Glia Medium (DMEM/F12 + GlutaMAX with 10% FBS and 1% PIS). HeLa and SY5Y cells were grown in DMEM with 10% FBS and 1% Pen/Strep (DMEM 5+). Transfection of positive controls (HeLa cells) were carried out with Lipofectamine 2000 (Invitrogen) as described below.
1.2 Engulfment Assays. SY5Y Cells were washed with Hanks Balanced Salt Solution (HBSS), then 2mL of the broad-spectrum tyrosine kinase inhibitor solution, Staurosporine (10uM), was added to each flask to induce apoptosis. The solution consisted of 30μL staurosporine in 2mL HBSS per flask. Flasks were then incubated for 3 hours at 37°C. SY5Y cells that had detached from the flask surface in the solution were collected, centrifuged for 5 minutes at 1500 rpm, quantified, then resuspended in DMEM. Apoptotic SY5Y cells were then distributed to each of the BV2 cell flasks to achieve a 1:1 ratio.
After respective time points of 0, 2, 4, and 24 hours, media was removed, the cells rinsed with PBS, 1mL ice-cold PBS was added, and a cell scraper was used to detach cells from the bottom of the flask. As a negative control, a 6cm dish of BV2 cells with no SY5Y cells added was collected at each endpoint. A 6cm dish of untouched BV2 cells for the duration of the experiment was collected as another negative control.
1.3 Protein Collection. Cells were collected after scraping and centrifuging at 2000 rpm for 3 minutes. Protein was isolated using 250uL of Radioimmunoprecipitation assay (RIPA) buffer, sonication, centrifugation at 14,000 rpm for 5 minutes at 4°C, and collection of supernatants containing the target proteins.
1.4 Bicinchoninic Acid Protein Assay (BCA Assay) and Western Blot (WB) Analysis. A BCA Assay was performed to quantify the collected proteins’ concentrations. A WB was conducted to assess the changes or presence of Jedi-1 in BV2 cells during different periods of engulfment. WB analysis was performed using sheep anti-Jedi-1 (1:5000) and mouse anti-GAPDH primary antibodies (1:10,000), and anti-sheep and anti-mouse HRP-conjugated secondary antibodies (1:5000).
- Assessing Engulfment of Apoptotic Cells by Transfected HeLa Cells
2.1 Cell Culture and Transfection. Approximately 25,000 HeLa cells were plated per chamber in the 4-well Chamber Slide™ System in DMEM 5+ media. The chamber slide containing HeLa cells was then placed at 37°C for 24 hours to allow the HeLa cells to settle and adhere. Before the transfection, the media was changed to serum-free and antibiotic-free DMEM.
The transfection solutions were prepared in a 1:1 ratio of DNA to Lipofectamine per the manufacturer’s recommendations. To visualize positive transfection of HeLa cells, the plasmid containing Jedi-1 includes a conjugated green fluorescent protein (GFP; Jedi-1-GFP). 50uL of Jedi-1-GFP plasmid/Lipofectamine solution or GFP plasmid/Lipofectamine solution was added to each chamber using a pipette. The chamber slides were placed at 37°C for 24 hours.
2.2 Engulfment Assay. Imaging: SY5Y cells were washed with HBSS (no ions) and stained with 5uM of CM-DiI for 10 minutes at 37°C, then 15 minutes at 4°C. SY5Y cells were washed again with HBSS. 3uM staurosporine was added to the SY5Y cells for 3 hours at 37°C. Supernatant containing SY5Y cells was collected, and the apoptotic cells were quantified. SY5Y cells were centrifuged for 5 minutes at 1500rpm and resuspended in DMEM. The SY5Y cells were distributed to the transfected HeLa cells to achieve a 1:1 ratio of SY5Y to HeLa cells. After respective time points, the media was vacuumed off, and cells were fixed in 4% PFA for 10 minutes at RT. The fixed wells were washed for 3×3 minutes in 1x PBS and then stored in fresh 1x PBS at 4°C.
WB: SY5Y Cells were washed with HBSS, and then 2mL of 10uM staurosporine solution was added to each flask. The solution consisted of 30uL staurosporine in 2mL HBSS per flask. Flasks were then incubated for 3 hours at 37°C. Supernatant SY5Y cells in the solution were collected, centrifuged for 5 minutes at 1500 rpm, quantified, then resuspended in DMEM. Apoptotic SY5Y cells were then distributed to each of the HeLa Jedi-1-GFP cell flasks to achieve a 1:1 ratio of SY5Y cells to HeLa cells.
After respective time points, media was removed, cells were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min.
2.3 Immunofluorescent Staining. The cells were permeabilized with PBS with 0.2% Triton-X100 (PBST) for 10 minutes at RT and then blocked with 5% BSA, 0.1% PBST for 1 hour at RT. Primary antibody (1:500 in PBS) was applied to wells, and the slides were placed at 4°C overnight. Primary was removed with 3×5-minute PBS washes. Secondary antibody (1:1000 in PBS) was applied for one hour, rocking at RT. Secondary was washed off with PBS for 3×5 minutes. Slides were mounted in ProLong Gold with DAPI for nuclear visualization.
2.4 Confocal Imaging. Images were collected in collaboration with the Vanderbilt Cellular Imaging Shared Resource core on a Zeiss LSM880 confocal microscope. 20x objective Z-stacks were taken of randomly selected fields of view, and engulfment was confirmed by orthogonal views in ImageJ.
2.5 BCA Assay and WB. BCA assay and WB protocol described previously for BV2 cells were applied to HeLa cells.
RESULTS.
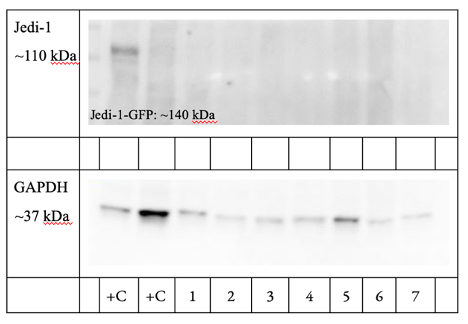
- Jedi-1 is not expressed endogenously in BV2 cells. The WB indicates that the Jedi-1 engulfment receptor protein is not expressed endogenously in BV2 cells and that its levels do not change upon exposure to apoptotic SY5Y cells (Figure 1; GAPDH as a control protein). The Jedi-GFP positive control was present, but there was no signal for the other positive control, HeLa Jedi-1-Flag. Future work will evaluate antibody specificity and continue to refine the protocol to reduce nonspecific binding.
Figure 1. BV2 Cells Do Not Strongly Express the Jedi-1 Protein and Jedi-1 Levels Do Not Change (n-2). Samples left to right: HeLa Jedi-1-Flag (+C), BV2 0 hour (1), BV2 2 hour (2), BV2 4 hour (3), BV2 24 hour (4), BV2 2 hour NO cells (5), BV2 24 hour NO cells (6), BV2 24 hour no cells no media change (7).
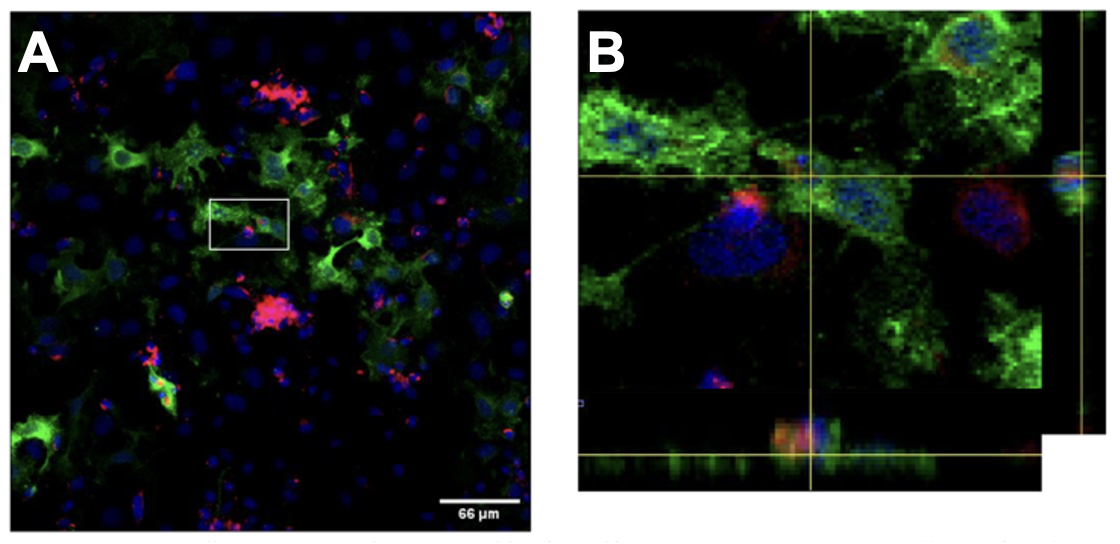
- Transfected HeLa cells phagocytose apoptotic SY5Y cells. After the transfection was deemed successful through immunofluorescent imaging, positively transfected HeLa cells (GFP) were cocultured with apoptotic SY5Y cells (CM-DiI) for 4 hours. Cells were then immuno-stained for GFP and imaged on a confocal microscope (Figure 2). If engulfment was occurring, it should be visible at four hours because microglia have been shown to initiate engulfment of their targets immediately upon contact with one another [9].
Figure 2. HeLa cells expressing Jedi-1 are capable of engulfing apoptotic neurons. HeLa cells transfected with Jedi-1-GFP (green) were cocultured with apoptotic SY5Y cells (red) for 4 hours. A: 20x max projection of confocal stack. B: Zoomed view of white box in A with orthogonal views showing internalized apoptotic cell.
This suggests that HeLa cells have gained the ability to recognize apoptotic SY5Y cell signals and engulf apoptotic debris after the transfection of the Jedi-1-GFP plasmid. However, it is important to note that engulfment needs to be quantified, and a negative control expressing GFP alone needs to be included. This work can be expanded upon to identify signaling partners downstream of Jedi-1 and is scalable to suit various endpoint readouts like WB, immunohistochemistry, RNA extraction, or mass spectrometry.
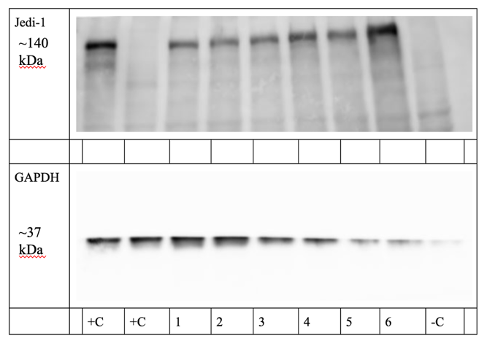
By collecting the proteins from transfected HeLa cells during the engulfment of SY5Y cells, we assessed the presence of Jedi-1 during different time points of engulfment. The WB indicated that HeLa Jedi-1-GFP cells exhibited sustained expression of Jedi-1 before, during, and after engulfment of apoptotic SY5Y cells (Figure 3). Even without introducing SY5Y cells, Jedi-1 expression is strong (Lanes 4 and 5, Figure 3). Notably, 24 hours after the introduction of SY5Y cells to the HeLa cells, Jedi-1 expression remains strong (Lane 4, Figure 3). After 24 hours, engulfment is likely to be over [9]; this shows that Jedi-1 expression does not disappear after engulfment of apoptotic debris in transfected HeLa cells.
Figure 3. HeLa cells transfected with Jedi-1-GFP show sustained Jedi-1 expression throughout engulfment (n=2). Samples left to right: HeLa Jedi-1-GFP (+C), HeLa Jedi-1-Flag (+C), 30 minutes (1), 2 hour (2), 4 hour (3), 24 hour (4), 2 hour NO SY5Y cells (5), 24 hour NO SY5Y cells (6), SY5Y cells (-C).
DISCUSSION.
We sought to identify a useful model method to study the signaling pathways downstream of Jedi-1 that are altering microglia phenotype. Our initial hypotheses were that (1) BV2 cells expressed Jedi-1 endogenously and Jedi-1 expression in BV2 cells would increase during the engulfment of apoptotic SY5Y cells, and (2) HeLa Jedi-1-GFP cells would be capable of phagocytosing apoptotic SY5Y cells.
In many neuropathologies, microglia appear beneficial as they exhibit an anti-inflammatory phenotype initially, but they later take on a detrimental pro-inflammatory phenotype, leading to further degeneration [10]. It is currently unknown if and how Jedi-1 participates in that phenotype change, but this work rules out BV2 cells as a useful model and identifies HeLa cells transfected with Jedi-1 as a potential model system to investigate this phenomenon further.
Our WB results show that BV2 cells are unlikely to express Jedi-1 endogenously, and Jedi-1 expression in BV2 cells is unchanged during the engulfment of apoptotic SY5Y cells (Figure 3).
Though BV2 cells have been deemed suitable as a replacement for primary microglia in previous studies [8], there are differences between BV2 cells and primary microglia. Because of the importance of microglia in neurodegeneration, toxicology, and immunity, the use of BV2 cells as substitutes for primary microglia in research has increased because of BV2 cells’ immortality, ease, and efficiency, as well as the effect of reducing the use of animals [10]. These results characterize a difference between BV2 cells and the cells they are meant to mimic (primary microglia). Our future directions include performing similar methods on primary mouse microglia to see whether Jedi-1 expression changes during the engulfment of apoptotic SY5Y cells. This information can inform other researchers of the differences between model microglia (BV2 cells) and primary mouse microglia in terms of Jedi-1 expression.
CONCLUSION.
We provide preliminary evidence to support the hypothesis that HeLa Jedi-1-GFP cells are able to phagocytose apoptotic SY5Y cells. There are obvious limitations in exclusively using transfected HeLa cells, as HeLa cells are not microglia-like cells. However, this model system isolated the Jedi-1 receptor to assess its independent functionality better and also provides a model that expresses Jedi-1 strongly. Our next step with this HeLa Jedi-1-GFP model system is to scale it to in vitro studies using isolated microglia and eventually to in vivo experiments. This will allow us to analyze the applicability of our findings to higher-scale model systems, subsequently revealing the validity of our findings in the CNS of living organisms. These findings are foundational for the continued investigation of the Jedi-1 receptor and to begin experiments more directly testing the involvement of phosphatidylserine in Jedi-1 facilitated engulfment. From this, we can conclude that HeLa cells are a useful model system to investigate signaling pathway(s) downstream of Jedi-1, including those that could alter microglia phenotype.
ACKNOWLEDGMENTS.
I would like to thank Matt Houpert, Dr. Bruce Carter, and everyone else at the Carter Lab for their guidance and expertise. I would also like to thank the SSMV for providing me with the opportunity to conduct this research.
REFERENCES
[1] J. Yuan, M. Lipinski, A. Degterev, Diversity in the Mechanisms of Neuronal Cell Death. Neuron.40, 401–413 (2003).
[2] Q. Li, B. A. Barres, Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol.18, 225–242 (2018).
[3] C. L. Hsieh et al., A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. Journal of Neurochemistry.109, 1144–1156 (2009).
[4] R. C. Paolicelli et al., Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science.333, 1456–1458 (2011).
[5] A. Sierra, O. Abiega, A. Shahraz, H. Neumann, Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci.7, 6(2013).
[6] H.-H. Wu et al., Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat Neurosci.12, 1534–1541 (2009).
[7] J. L. Scheib, C. S. Sullivan, B. D. Carter, Jedi-1 and MEGF10 Signal Engulfment of Apoptotic Neurons through the Tyrosine Kinase Syk. Journal of Neuroscience.32, 13022–13031 (2012).
[8] A. Henn, The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX, 83–94 (2009).
[9] A. Sierra et al., Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell.7, 483–495 (2010).
[10] X. Hu et al., Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke.43, 3063–3070 (2012).
Posted by John Lee on Tuesday, May 30, 2023 in May 2023.
Tags: HeLa, Jedi-1, microglia, Neuroscience