Cortical Evoked Potentials Identify Compensatory Effects of Attention on Auditory Processing in Children and Adults
ABSTRACT
Children and adults find listening in noise to be difficult; however, children are more prone to struggle as their auditory processes are less mature, and they are exposed to noisier environments. This study examined the effects of noise, attention, and age on speech processing in children (9-13 years) and adults (21-25 years). Differences in brain responses to speech presented in quiet and in noise with attention directed toward or away from the sounds were measured using Event-Related Potentials (ERPs). While noise reduced the detectability of speech in all participants (diminished N1 response amplitude) during passive listening, active attention provided compensatory effects for the effects of noise neural processing. This is suggested by the fact that attention affects were only present in noise. These findings suggest that the initial auditory process of children (9-13) is equally as mature as adults (21-25).
INTRODUCTION.
In a world with many competing sensory stimuli, people find it difficult to differentiate between relevant and irrelevant auditory information in the presence of noise (background sounds). Children may struggle more as they are less experienced listeners, their auditory system is less mature [1], and they are often exposed to noisier environments such as classrooms and playgrounds. Most prior studies of auditory processing have focused on adults [2-4]. Research involving children is limited, and there are even fewer studies that compare the two age groups [5,6]. Children and adults may rely on different auditory processes when attentive due to their diverging age and level of maturation, however, the degree to which they differ from one another is uncertain [5]. The purpose of this study is to discern whether attention to speech affects the early auditory sensory processes of adults and children similarly in quiet and noisy conditions.
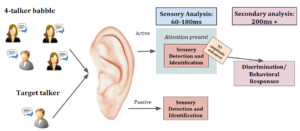
Electroencephalograms (EEGs), which measure the electrical activity of the brain, offer an effective means to evaluate sensory and attentional processes. The amplitude of the N1 potential, a negative peak found between 60 to 180 msec after stimulus onset, reflects processing of sensory stimuli (Figure 1). Previous studies reported that with a slow stimulus presentation rate, children and adults generate similar auditory N1 amplitudes in quiet [4]. However, when listening in noise, the N1 amplitude decreases [7]. Thus, we predict no differences between children and adults in the N1 response to speech in quiet, while the extent of N1 amplitude reduction in noise may vary with age. Additionally, the N1 amplitude becomes larger when attention is directed to the stimulus than during passive exposure [2], suggesting enhanced sensory representation [1]. We hypothesized that attention to stimuli will improve speech processing in noise in both age groups; however, we anticipated that children would have poorer performance and lower N1 amplitudes in comparison to adults. Our hypotheses are reflected in Figure 1 as it displays the auditory process and what occurs when presented with the 4 conditions established in this study.
Figure 1. A graphic description of the auditory process and the processes that occur amongst conditions (i.e. both in noise and in quiet, one may pay attention; in active noise, N1 amplitude is enhanced by the presence of attention; in passive noise, N1 amplitude is diminished without the presence of attention).
To gain a more comprehensive understanding of the differences of the speech processing in children and adults, accuracy and speed of behavioral responses were measured to determine if children perceived the listening task to be equally difficult as compared to adults. We predicted that adults and children would find the tasks to be similarly difficult in quiet, but not in the noisy condition. Overall, we predicted that children would find the task to be more challenging than the adults.
MATERIALS AND METHODS.
Participants.
Fifty-eight participants (aged 7-25) with normal cognitive and hearing abilities were recruited from the campus area to participate in the more comprehensive study completed on listening in noise. The guardians of the children participating as well as the adult participants provided a written informed consent. All procedures were approved by the Institutional Review Board.
Data for a subset of 15 children (3 females) age 9-13 years (M = 11.42, SD = 1.34) and 15 adults (9 females) age 21-25 years (M = 23.53; SD = 1.58) were used to address this study’s hypotheses. There were no significant differences found in the data between sexes. Ages 9-13 years were chosen as the youngest group with the classic N1 response, while children 8 years old and younger display a different ERP morphology, complicating the comparison with adults [6]. Ages 21-25 years represented the youngest group when structural and functional maturation of the brain is fully completed [6].
Stimuli.
Two syllables, /da/ and /ga/, naturally spoken by the same female talker, served as the stimuli in the quiet condition. These syllables were chosen as their perception is affected by background babble noise. For the noise condition, the stimuli were presented against the background of a continuous four-talker babble, composed of three female and one male voice. The speech tokens (the syllables presented in all of the study’s defined conditions) were presented via insert earphones at 75 dB SPL and the noise at 60 dB SPL, creating a +15 dB signal-to-noise ratio.
Procedures.
Participants completed the Kaufman Brief Intelligence Test (KBIT-2; Kaufman & Kaufman, 2004) to confirm typical cognitive functioning, and a standard hearing screening at 15 dB HL for octave frequencies ranging from 250 to 8000 Hz to verify normal hearing. Participants had normal hearing as verified by a standard hearing screening at 20 dB HL for octave frequencies ranging from 1000-8000 Hz. Next, a 128- channel EEG net (EGI, Inc; Eugene, OR) was placed on their head, and the stimuli were delivered using ER-3A insert earphones. NetStation 5.3 software was used to collect the EEG data with 250 Hz sampling rate. E-prime 2.0 (PST, Inc; Pittsburgh, PA) controlled stimulus presentation. Stimuli were delivered using an oddball paradigm, in which a series of standard sounds (80%, 160 trials) is interrupted infrequently (20%, 40 trials) by a target stimulus. Syllable assignment to standard versus target was counter-balanced across participants. All subjects completed four blocks of trials involving passive listening or active sound discrimination, each performed in quiet and in noise conditions. In the passive condition, participants watched a silent film and were instructed to ignore any sounds they heard. In the active condition, participants were asked to pay attention and identify each syllable heard by pressing the corresponding button on a hand-held response box. Accuracy and speed were emphasized for this task. No silent movie was played during the active condition. The order of these conditions was counterbalanced across participants.
Analysis.
Event-Related Potentials.
The raw EEG data were filtered to exclude frequencies outside of the 0.1-30 Hz range, segmented on syllable onset to include a 100 msec prestimulus baseline and 900 msec post-stimulus interval. Following the standard procedures for ERP analysis, the raw EEG data was filtered in this manner. This step allowed for isolation of the neural signals characterizing auditory processing of speech stimuli. The individual trials were reviewed to remove any artifacts (facial/muscle movement that obstruct the ERPs) using automated tools in NetStation 5.3 software, followed by manual review. Artifact-free trials were averaged together to isolate stimulus-related activity that was consistently present for each trial. The ERPs were referenced to the average of all electrodes and baseline corrected. Mean N1 amplitude was obtained for the vertex electrode cluster by averaging values across consecutive time points within 60-180 msec. The resulting values were submitted to a 3-way repeated measure analysis of variance (ANOVA). Within-subject factors included Task (2: active, passive) and Condition (2: noisy, quiet). The between-subjects factor was Age (2: children, adults). Afterwards, a paired post-hoc t-test or planned comparison test were completed to determine the direction of the significance for each main effect and interaction drawn from the ANOVA (a statistical test that is considered the accepted approach to analyzing ERP data to accommodate normalcy) [8]. As multiple statistical tests were completed on the same set of data, the P-values were corrected for multiple comparisons in the post-hoc test using the Bonferroni test.
Behavioral Measures.
The button-press data from the active conditions was used to calculate a hit rate, or a percentage of correct speech sound identification for standard and target stimuli. Additionally, the response times in msec were recorded. Mean accuracy and median response time were used to evaluate group differences in task performance. A single repeated measures two-way ANOVA with Age (2) x Condition (2) factors and planned paired t-test were used to identify differences between perceived level of difficulty for children and adults. Because behavior was measured using the accuracy and response time for button selection only during the active condition, task (passive vs. active) was not included as a factor for this specific test.
RESULTS.
Event-Related Potential Results.
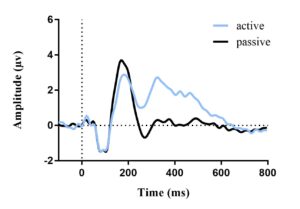
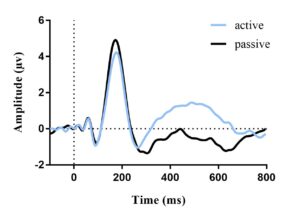
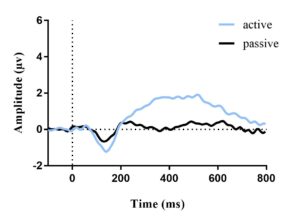
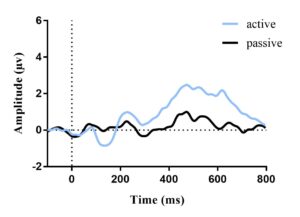
The average ERP waveforms for each condition and age group are presented in Figures 2 and 3. In each figure, an N1 amplitude can be observed within the 60-180 msec window. This reveals that both age groups have consistent waveform morphology.
A)
B)
Figure 2. Average ERP waveform for adults (a) and children (b) in quiet elicited by target stimuli; no difference in N1 amplitude between active and passive listening. Vertical dotted line represents stimulus onset.
A)
B)
Figure 3. Average ERP waveform for adults (a) and children (b) in noise elicited by target stimuli; active amplitude of N1 found to be greater than passive. Vertical dotted line represents stimulus onset.
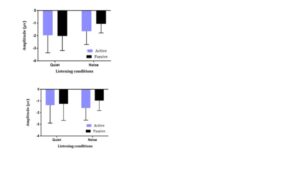
The repeated measures ANOVA identified a main effect of Task, F(1,28) = 4.956, p = 0.034 (Figure 4), revealing that across both age groups, the N1 amplitude in the attentive condition was larger than in the passive condition. Although the main effect of Condition was not significant, F(1,28) = 3.506, p = 0.072, a significant Task x Condition interaction was found; F(1,28) = 5.043, p = 0.033 (Figure 4). Planned comparisons examining the effects of noise indicated that N1 amplitudes in quiet and noise were not significantly different in the active task, t(29) = 0.163, p = 0.871 (Figure 2, Figure 3). Conversely, during the passive task, N1 amplitudes in noise were significantly smaller than those in the quiet condition, t(29) = 2.963, p = 0.006 (Figure 4). The post hoc t-test of the task effect indicated that N1 amplitudes in the passive and active tasks were not significantly different in the quiet condition, t(29) = -.0152, p = 0.881 (Figure 2, Figure 3); however, during the noise condition, N1 amplitudes in the active task were greater than in the passive task, t(29) = -3.205, p = 0.003 (Figure 2, Figure 3). The effect of age was not significant, F(1, 28) = 1.238, p = 0.275 (Figure 4).
Figure 4. Means and standard deviations of N1 amplitudes for the combined sample (N = 30; children: N = 15; adults: N = 15) of adults (a) and children (b) across conditions; in quiet, there is no difference between the active and passive conditions; for noise, active is greater than passive (F(1,28) = 4.956, p = 0.034).
Behavioral Results.
Response Accuracy.
There was a main effect of Condition, F(1,28) = 5.72, p = 0.025, driven by lower accuracy in noise compared to quiet. There was no significant Condition x Age interaction, F(1,28) = 3.730, p = 0.064, yet planned comparisons within each age group revealed that children demonstrated poorer performance in the noise compared to the quiet condition, t(14) = 2.226, p = 0.002. However, the adults maintained comparable performance between the two conditions, t(14) = 0.807, p = 0.007. The mean and standard deviation values of response accuracy for children are as follows: noise: M = 0.97; SD = 0.080; quiet: M = 0.98; SD = 0.069. As for adults, the values were: noise: M = 0.99; SD = 0.048; quiet: M = 0.99; SD = 0.041.
Response Time.
The main effect of Condition, F(1,28) = 16.534, p < 0.001 revealed longer response times in noise than quiet. Results showed no interaction between condition and age, F(1,28) = 0.246, p = 0.624. Planned comparisons within each age group showed that response times were longer in noise than in quiet for children, t(14) = -2.757, p =.0015, and adults, t(14) = -3.177, p = 0.007. The mean and standard deviation values of response time for children are M = 569.23 ms; SD = 93.23 ms; quiet: M = 569.23 ms while for adults, the results were M = 436.13; SD = 74.21.
Behavioral Correlations.
This exploratory analysis was completed to determine if a relationship between N1 potentials and behavioral accuracy exists. N1 amplitudes and response accuracy in the active conditions were not significantly correlated in quiet (children: r = 0.330, p = 0.230; adults: r = -.0428, p = 0.112) or in noise (children: r = 0.087, p = 0.757; adults: r = -.0039, p = 0.890).
DISCUSSION.
The goal of this study is to determine the effects of age, task, and condition on identification of speech-in-noise. This study is the first to examine all three variables on the N1 potential, which reflects processing of sensory stimuli and attention. Testing the two groups will yield results that will answer the long-asked question of how adults and children differ in their early auditory process.
Behavioral Testing.
Analysis of accuracy and reaction time identified no significant age differences, suggesting that children and adults found the sound discrimination task to be of similar difficulty. Lower accuracy and longer response times in noise than quiet for both children and adults were consistent with prior evidence that noise has a negative effect on speech processing [7].
Event-Related Potentials.
In the quiet condition, no significant differences were found between the active and passive N1 amplitudes for both children and adults. These results are consistent with previous findings claiming that when sound detection and discrimination should be easy, there would be no significant benefit from attention [4,5]. Conversely, the effect of attention was present in the noise condition, where passive N1 amplitudes decreased in comparison to the active N1 amplitudes. Again, these results were similar for both children and adults, supporting the hypothesis that the noise paradigm would prove to be difficult, and therefore cause a decrease in N1 amplitude without the presence of attention.
Moreover, the addition of noise reveals distinct differences between the N1 potentials for children and adults [4,7]. In noise, sensory processing is disrupted, reflected by a decreased N1 amplitude. However, attention facilitates sensory processing, which amplifies the N1 and makes it similar to the amplitudes found in the quiet condition, thus compensating for the effects of noise.
This is the first to examine the effects of noise in each age group, and the lack of age-related differences contradicted the hypothesis that differences would be present between age groups. Since both age groups display similar results in all four conditions, it can be concluded that attention affects sensory processing in similar ways in 9-13-year-old children and in 21-25-year-old adults. As the N1 potential represents initial identification of stimuli, it is inferred that by 9-13 years of age, basic auditory processes are already mature, and differences in behavioral performance are more likely to be attributed to attentional differences [9]. Future studies using the same methodology will examine the impact of age and noise on later stages of auditory processing to gain a larger picture of the development of the entire auditory process. However, in order to fully understand these segments of the process, some adjustments should be considered for future studies: the statistical power should be increased by an increase in sample size; the age range should be expanded to include younger children such as infants and toddlers to capture the developmental course of the auditory processes and the compensatory effects of attention; other attention-specific neural responses, such as the P3 potential, should be considered to yield new information regarding higher-order auditory processing in noise.
ACKNOWLEDGMENTS.
This work was supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH (Gustafson and Key) and by the Council of Academic Programs in Communication Sciences and Disorders. Special thanks go to Dr. Key, Dr. Gustafson, Dorita Jones, Javier Santos, all the participants, Dr. Muller and the SSMV, as well as the Vanderbilt Kennedy Center. The content is solely the responsibility of the authors and does not represent the official views of the National Center for Advancing Translational Sciences or the National Institute for Health
REFERENCES.
- S. Anderson, B. Chandrasekaran, H.G. Yi, & N. Kraus, Cortical-evoked potentials reflect speech-in-noise perception in children Euro. Jour. Of Neuro. 32, 1407-1413 (2010).
- S. A. Hillyard, R. F. Hink, V. L. Schwent, & T. W. Picton, Electrical signs of Selective Attention in the Human Brain. Science. 182, 177-180 (1973).
- R. Näätänen, A. Gaillard, & S. Mäntysalo, Early selective attention effect on evoked potential reinterpreted. Acta Psychologica. 42, 313-329 (1978).
- R. Parasuraman, Effects of information processing demands on slow negative shift latencies and N100 amplitude in selective and divided attention. Biological Psychology, 11, 217-233 (1980).
- S. Maatta, A. Paakkonen, P. Saavalainen, J. Partanen, Selective attention event-related potential effects from auditory novel stimuli in children and adults. Clinical Neurophysiology. 116, 129 – 141 (2005).
- D. Coch, L. Sanders, & H. Neville, An Event-related Potential Study of Selective Auditory Attention in Children and Adults. Journal Of Cognitive Neuroscience. 17, 605-622 (2005).
- C. J. Billings, K. O. Bennett, M. R. Molis, & M. R. Leek. Cortical encoding of signals in noise: effects of stimulus type and recording paradigm. Ear and Hearing, 1 (2010).
- J. Dien, A. Santuzzi, Application of repeated-measures ANOVA to high-density ERP Databases: Review + Tutorial. ResearchGate. (2009).
- A. Key, G. Dove, & M. Maguire. Developmental Neuropsychology. 27, 183-215. (2005).
- A. S. Kaufman, N. L. Kaufman, Kaufman Brief Intelligence Test, Second Edition (KBIT-2). (2004) https://www.pearsonclinical.com/psychology/products/100000390/kaufman-brief-intelligence-test-second-edition-kbit-2.html.
Posted by John Lee on Tuesday, December 22, 2020 in May 2018.
Tags: auditory processing, event-related potentials, N1, Speech-in-noise perception