Breast Cancer Metastasis to the Bone Microenvironment
ABSTRACT
70% of advanced breast cancer cases involve metastasis to the bone. The intricate microenvironment of the bone is conducive to tumor cells that have detached from the mammary epithelium. Bone homeostasis is disrupted by the vicious cycle of metastasis as the bone is overly degraded and more prone to pathologic fractures. The adipocytes in the bone marrow provide a rich energy source for cancer cells, which facilitates rapid proliferation. All bone marrow lacks oxygen, but in patients with breast cancer, hypoxia is further induced. This activates the transcription factor, Hypoxia Inducible Factor 1, which promotes the epithelial to mesenchymal transition. Increased matrix stiffness also promotes the malignant phenotype through a mechanotransduction cascade. This review will elaborate on three different aspects of the bone microenvironment: the adipocyte niche, hypoxia, and the biophysical cue of stiffness, and discuss the application of 3D in vitro hydrogels.
INTRODUCTION.
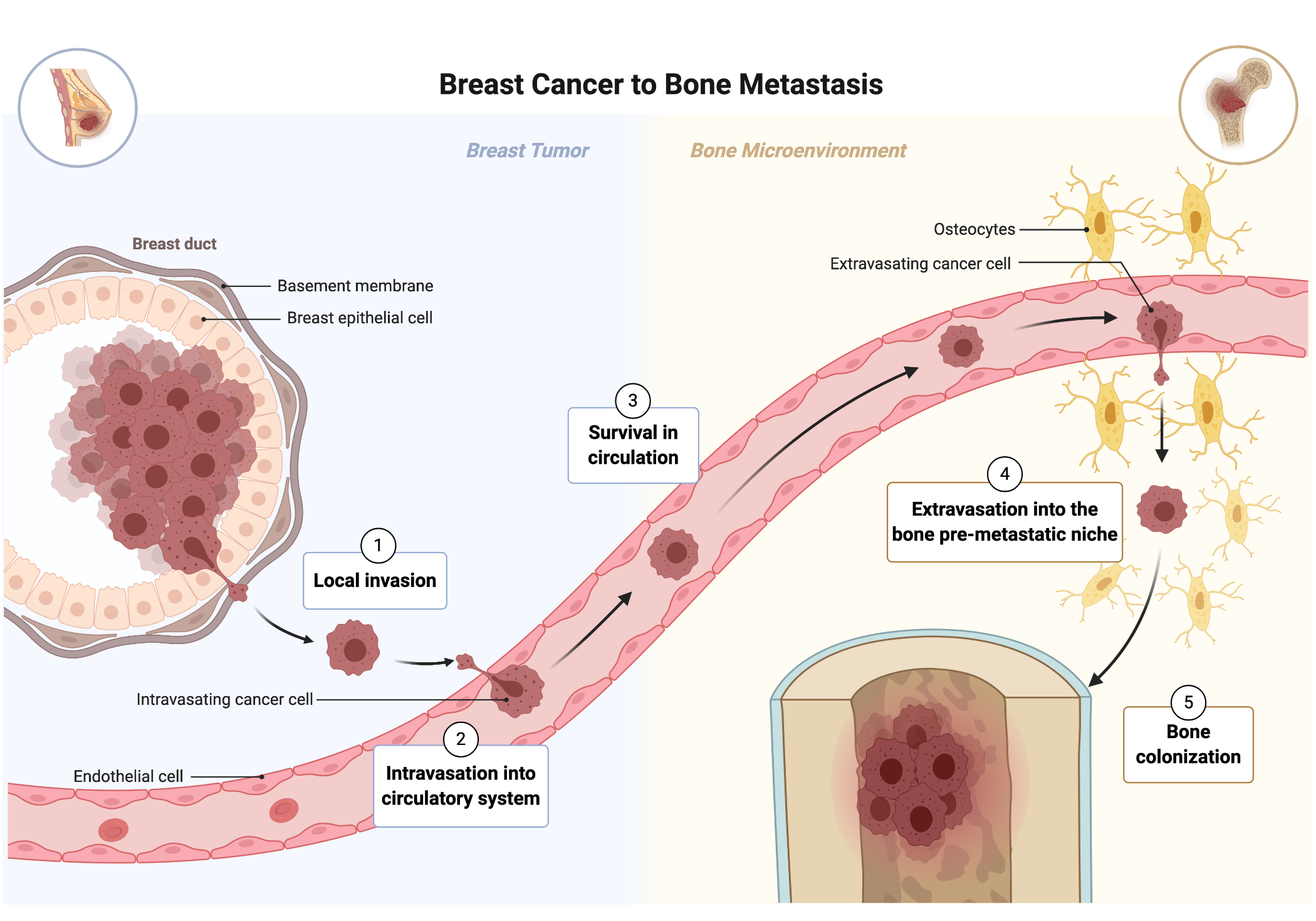
Breast cancer is the most common cancer diagnosed [1]. More than 90% of breast cancer-related deaths are attributable to metastasis [1]. The metastatic cascade unfolds when tumor cells detach from the primary breast region, traverse past basement membranes, and successfully invade other parts of the body. This complex, multistep process includes (1) local invasion where the tumor cells break away from the primary tumor; (2) intravasation into the circulatory system; (3) survival in circulation; (4) extravasation of the tumor cell into the target organ’s pre-metastatic niche; (5) colonization of the distant site [5] (Fig. 2). The brain, lymph nodes, and liver are common sites of metastasis, but the bone attracts the highest concentration of cancer cells [2]. The bone microenvironment consists of the exterior, rigid cortical bone at a stiffness of 20,000 kPa and spans into the softer, sponge-like tissue of the bone marrow at a stiffness of 0.5-1 kPa [2]. The endosteum is a thin vascular membrane that lines the trabecular and endocortical bone surfaces.
Figure 2. The steps of breast cancer metastasis. Adapted from BioRender.com
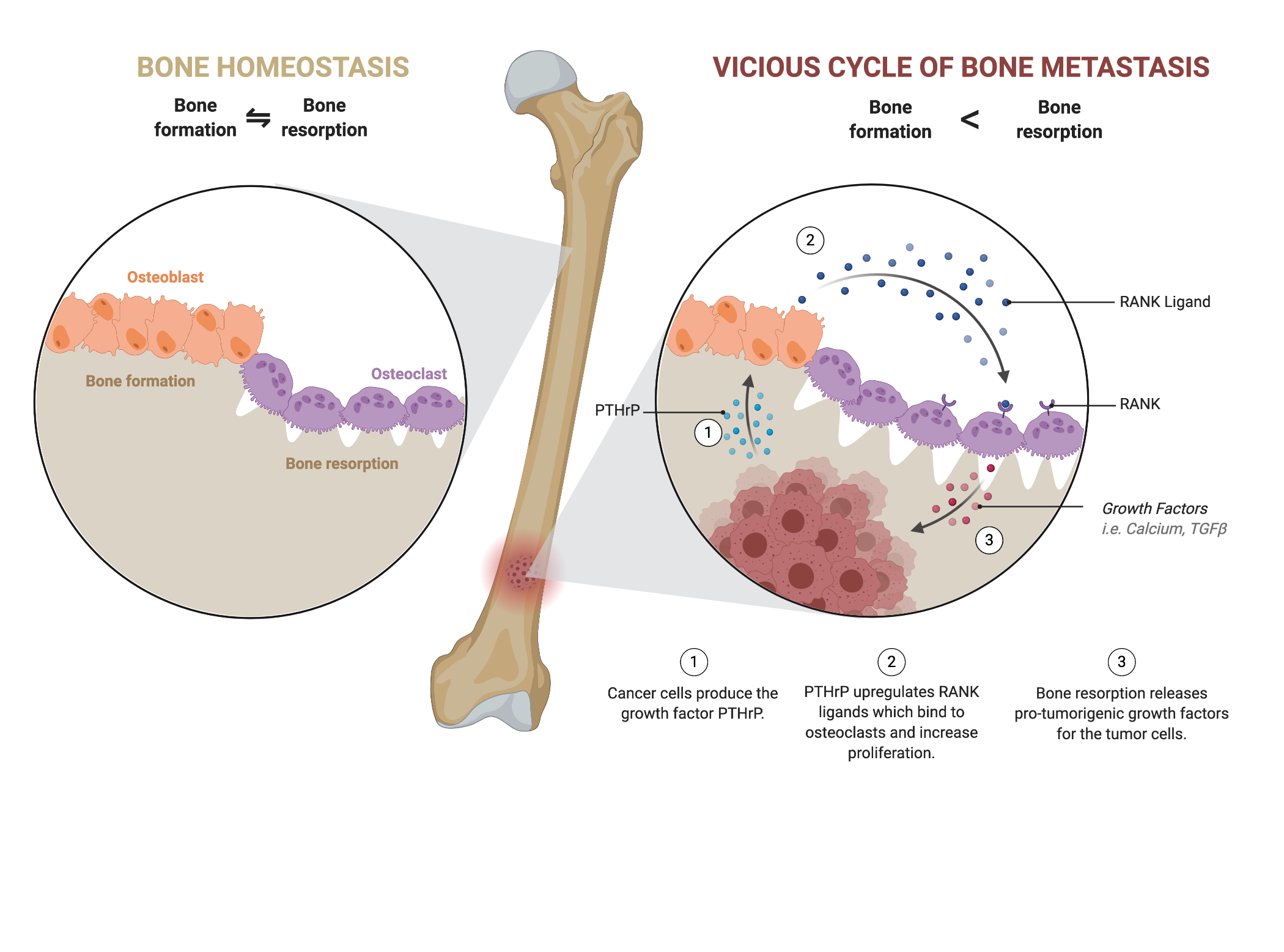
In the endosteal niche, the interaction between tumor cells and cortical bone constitutes the vicious cycle of bone destruction (Fig. 1). Osteoblasts create new bone and are coupled with osteoclasts that degrade the bone to regulate bone homeostasis. Tumor cells disrupt the bone-remodeling homeostasis by upregulating parathyroid hormone-related peptide which upregulates the receptor activator for nuclear factor kappa-b (RANK) ligand [3]. This upregulation causes osteoclasts to over degrade the bone matrix, which induces critical pain, hypercalcemia and pathological fractures along with a higher morbidity rate [1] [2] [6]. The osteoclast releases transforming growth factor β (TGFβ) and calcium that continues to fuel the recruitment of tumor cells, establishing the vicious cycle of bone destruction [3].
Figure 1. In the vicious cycle of bone metastasis, bone homeostasis is disturbed as bone resorption is upregulated. Overactive osteoclasts cause weak bones prone to pathologic fractures and are indicative of a poorer prognosis. Adapted from BioRender.com
Less than 0.01% of tumor cells are able to successfully metastasize, yet 70% of advanced breast cancer cases have reported bone metastases [6]. A study by the Surveillance, Epidemiology, and End Results Program concluded that all breast cancer subtypes, and especially the HR+/HER2+ (luminal-HER2) subtype, are prone to initiating bone metastasis [12]. There is a significant discrepancy between the statistically low probability of a successful distant metastasis and the frequent occurrence of bone metastasis observed in the clinic.
Since Stephen Paget’s proposal of the pivotal “Seed and Soil” hypothesis, research has continued to support that cancer cells, or the “seeds”, require a specific organ microenvironment, or “soil” in order to successfully metastasize. This hypothesis has been instrumental to metastatic organotropism, and present-day research on the “fertile soil” of distant metastatic sites continues to corroborate Paget’s hypothesis. The diverse bone microenvironment has many characteristics that establish it as a premetastic niche. The tumor microenvironment created from the premetastic niche is different from healthy tissue. This review will elucidate the unique interplay between the adipocyte niche, hypoxia, and the biophysical cue of stiffness across the cortical bone and bone marrow that make the dynamic bone microenvironment a prime breeding ground for breast cancer cells.
EPITHELIAL TO MESENCHYMAL TRANSITION.
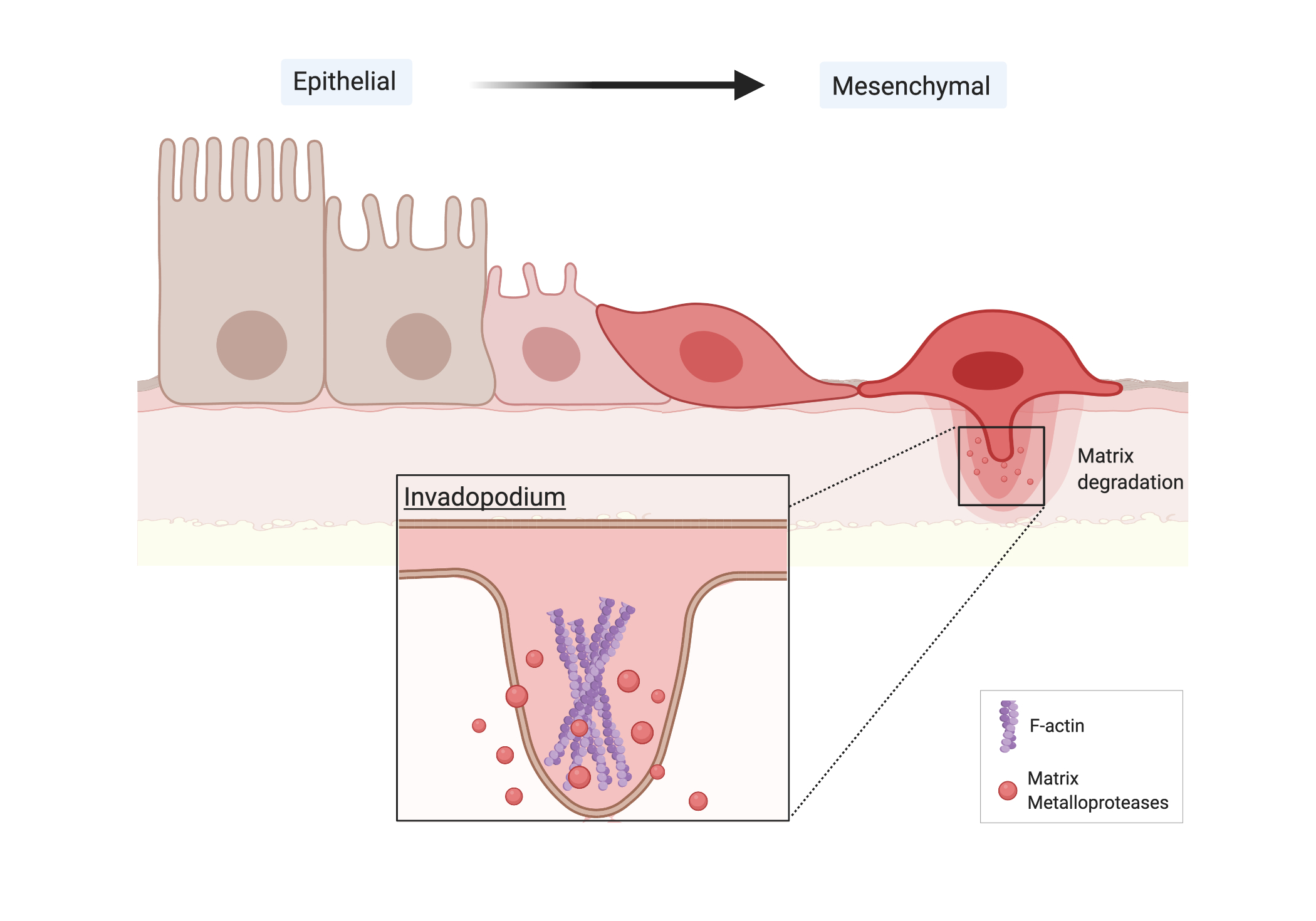
The epithelial to mesenchymal transition (EMT) plays a significant role in promoting the initial step of metastasis [4]. Primary epithelial tumor cells lose polarity and dissolve tight cell-cell adhesions to acquire a higher migratory capacity and mesenchymal morphology. For instance, E-cadherin, a transmembrane glycoprotein that organizes epithelial cellular adhesion, is downregulated to increase cancer cell motility and invasiveness at sites of EMT [4] (Fig. 3). Simultaneously, mesenchymal markers like vimentin and fibronectin are upregulated [4]. Transcription factors like Snail, Slug, ZEB1, ZEB2, and Twist1 are activated to directly bind and suppress the E-boxes of E-cadherin promoters [8]. Once the cancer cells disintegrate the intercellular E-cadherin adhesions, they have to traverse tight tissue barriers. After the circulating tumor cells (CTCs) have detached from the primary tumor, they become mesenchymal cells. Aggressive tumors release thousands of malignant, circulating tumor cells into the bloodstream daily, but only 0.01% of the CTCs initiate a distant metastasis [6] [7]. EMT is an important milestone of metastasis.
Figure 3. Cancer cells undergo the EMT and form invadopodia. Created using BioRender.com
For metastasis to occur outside of the primary breast tumor, cancer cells intravasate into tightly confined tissue barriers of the basal membrane. The mesenchymal cells exhibit a more invasive phenotype with activated transcription factors and invadopodia that enable them to permeate basement membranes and circulate to distant parts of the body [9]. The cancer cells must locally degrade the physical barrier presented by the extracellular matrix (ECM). Invadopodia are specialized actin-based membrane protrusions that develop in the mesenchymal cells and initiate degradation of the ECM by secreting proteases. They are F-actin rich plasma-membrane protrusions with a diameter of 8 μm and a depth of 5 μm [9]. In mesenchymal cells, invadopodia release Matrix metalloproteinases (MMPs) to lyse the ECM components and allow the cell to migrate through [9]. MMPs are from the zinc-dependent family of endopeptidases that help with physiological processes like wound healing, uterine involution, and organogenesis. The CTCs have completed intravasation after they enter the basal membrane and circulation.
As cancer cells undergo reprogramming of their lipid and glucose metabolism, their chances of survival increase. The Warburg Effect, presented by Otto Warburg in 1920, refers to the tendency of cancer cells to favor glycolysis over oxidative phosphorylation even in sufficiently normoxic environments in the presence of oxygen [12]. This aerobic glycolysis is a hallmark of cancer cell metabolism because it provides higher cancer cell survival and resiliency in homing to the premetastatic niche. Oxidative phosphorylation (OXPHOS) is an aerobic process that produces ATP from the transfer of electrons. Noncancerous cells depend on oxidative phosphorylation under normal physiologic conditions. In contrast, glycolysis is the anaerobic systematic breakdown of glucose into 2 pyruvates and ATP and NADH for energy. OXPHOS creates energy more efficiently, yet cancer cells utilize glycolysis regardless of the surrounding oxygen concentration. Cancer cells rewire their metabolism to use aerobic glycolysis because the high glucose concentration in the tumorigenic environment provides important intermediates for the pentose phosphate pathway. In particular, aerobic glycolysis creates amino acids, ribose sugars from nucleotide synthesis, and glycerol and citrate from synthesized lipids [10]. In addition to the formation of these intermediary substances, the high concentration of glucose initiates a high rate of glycolysis that is more efficient and produces more ATP than OXPHOS [10]. OXHPOS has also been shown to increase cancer cell apoptosis which further supports the underlying benefits of glycolysis that are conducive to tumorigenesis [11].
The adipocyte niche also serves an integral role in establishing the larger pre-metastatic niche of the bone marrow that recruits breast CTCs. Adipocytes, or fat cells, are prominent in the bone marrow, and constitute the yellow fat recognized as bone marrow adipose tissue (BMAT). The adipocyte composition is dynamic and changes with factors such as obesity, age, diet changes, and radiation. BMAs supply exogenous lipids that the breast cancer cells need. Studies on prostate cancer, leukemia, ovarian cancer, and multiple myeloma cells corroborate the idea that cancer cells exhibit enhanced lipid uptake in the presence of BMAs, and marrow adipocytes also upregulate lipases, the pancreatic enzyme that catalyzes breakdown of fatty acids and glycerol for the aerobic glycolytic pathway [13] [14] [15]. The studies suggest that adipocytes constitute a large portion of tumorigenic environments and provide the lipid exchange for fueling various cancer cells. As tumor cells rely on the exogenous lipids from the BMAs, lipid signaling manipulates the mitochondrial homeostasis of cancer cells to promote malignancy.
HYPOXIA
Hypoxia, or oxygen deficiency, exacerbates the harsh metabolic conditions of the bone marrow metastatic niche. 25-40% of invasive breast cancer cases reported the cancer cells had metastasized to hypoxic regions of the bone microenvironment [20]. The median partial pressure of oxygen in normal human breast tissue is 65 mmHg while the pO2 of human breast cancer tissue is a median of 10mmHg [17] [18]. pO2 of human bone marrow ranges from 9.9-31 mmHg [19]. The bone marrow is a distinctly hypoxic region that is conducive to disseminated tumor cells (DTCs) and is associated with inducing more aggressive cancer cell phenotypes [20].
The hypoxic region of the bone marrow activates hypoxia-inducible-factors (HIF) which are crucial in forming the pre-metastatic niche. HIFs are responsible for initiating cells’ adaptations to oxygen depleted environments. HIF-1 is the heterodimer, bioactive form that consists of alpha and beta subunits. Expression of the alpha subunits is induced by pO2 fluctuations, and the beta subunits are constitutively (?) expressed in the tissues [20]. HIF-1 provokes EMT by regulating EMT-associated transcription factors or repressors. It has transcriptional control of E-cadherin, SNAIL, ZEB1, and TWIST which were discussed in section 1.2 as initiators of EMT, the initial step of metastasis. These factors are overexpressed in hypoxic conditions. Hypoxia also causes an overexpression of ecto-5’-nucleotidase (CD73) [16]. CD73 regulates the interactions among T cells, endothelial cells, and ECMs to mediate metastasis and angiogenesis of carcinomas. An adenosinergic cascade is stimulated as hypoxia causes an overexpression of HIF and, subsequently, CD73. CD39 is an integral membrane protein that plays a strategic role with CD73 in order to calibrate the metabolism and signals sent to immune cells [16]. In particular, CD73 and CD39 initiate phosphohydrolases of ATP which provides adenosine. The adenosine is detached and freed into the extracellular space, where the extracellular adenosine signals are immunosuppressive. Through this transduction, CD73 is also a strong immune regulator [16].
MECHANOSENSING AND STIFFNESS
The ECM surrounding breast cancer cells is hypothesized to play a pivotal role in tumorigenesis as it provides the necessary physical and biochemical factors for metastasis. The ECM consists of insoluble proteins, glycosaminoglycans, and proteoglycans which provide the three-dimensional (3D) structural support for cells [24]. Phenotypically, cancer tissue differs from normal tissue because it exhibits an altered ECM density and composition. Stiffness is a measure of the rigidity of the tightly confined extracellular matrices. For initial screenings of breast cancer, physicians utilize mammography scans and palpitations to identify abnormally dense tissue [2]. In addition, the tumor stroma is remodeled throughout tumor progression as the cancer cells exert traction forces on the ECM and locally degrade the matrix to increase their motility and leave the primary tumor or enter the bone metastatic site [21]. This tissue remodeling causes the tumor stroma to have varied matrix stiffness and composition, which influences invasion and growth of DTCs. The cortical bone is a favorable environment to DTCs because it has a stiffness of ~20,000 kPa [2]. Invasion of the bone also causes the matrix to become stiffer, which is significant to oncology because stiffer tissue has been implicated to induce more malignant phenotypes [22].
To investigate the critical cell-ECM interactions and compositions, researchers create 3D in vitro hydrogels to mimic the bone microenvironment [21] [22]. These Alginate-Matrigel models provide precise control of chemical and mechanical properties. Stiffness can be modulated independent of other factors, like cell polarity and alterations of the basement membrane physiology. In particular, 3D hydrogels with interpenetrating networks of calcium and alginate mimic tumorigenic environments [22]. The stiffness of the model ranges from 30 to 300 Pa which renders the 3D hydrogel a novel technique because previous models have not successfully recapitulated this anatomically precise range of stiffness. 2-dimensional models do not accurately recapitulate 3D human in vivo conditions [23]. The models also modulate smaller ranges of stiffness that do not accurately recapitulate in vivo bone stiffness [24]. A rheometer measures stiffness by applying force to the hydrogel sample and assessing its deformation response as the sample loses its capacity to restore its original structure. Once the models were tested in the rheometer, they were compromised and permanently deformed before reaching the stiffness of bone. MCF10A breast cancer cell line in stiff 3D hydrogels underwent EMT at a higher rate. These results further corroborate the hypothesis that the increased stiffness signals the mechanotransduction cascade towards tumorigenesis.
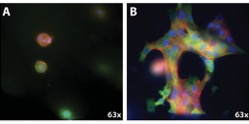
To view the cells growing in the models, researchers use a fluorescence microscope. In Figure 4, 4T1 triple negative breast cancer cells are GFP labeled (green) and cultured in Alginate-Matrigel hydrogels at (A) 0.5 kPa and (B) 10 kPa for 7 days. Cytoskeletal F-actin and nuclei were visualized using phalloidin (red) and DAPI (blue). In the higher stiffness (B), the cells exhibit a more malignant phenotype; they are more proliferative, and the cells display a striated, mesenchymal structure with more protrusions and higher invasive capacity (Fig. 4). 3D hydrogels are a successful technique; they effectively modulate stiffness independent of other biological factors which helps support the hypothesis that malignant phenotypes are induced and promoted by the independent variable of stiffness.
Figure 4. TNBC cells were cultured in alginate-Matrigel hydrogels of (A) 0.5 kPa and (B) 10 kPa stiffnesses. Courtesy of the Rafat Lab.
CONCLUSION
As Dr. Stephen Paget proposed, the fertile soil of the bone microenvironment is conducive to metastasis of the cancer cell seeds. The dynamic and diverse bone microenvironment makes it a prime breeding ground for circulating tumor cells. The bone marrow adipocytes bolster the cancer cells’ metabolism in the hypoxic region as the cells initiate aerobic glycolysis. In addition to the biological factors that promote tumorigenesis, the biophysical factor of stiffness also is pivotal to priming the “fertile soil” of the bone. Higher stiffnesses cause more mesenchymal, malignant cells that traverse through membranes with their invadopodia and MMPs. The physical interactions and biochemical aspects play equally important roles in metastasis. More research and utilization of 3D models has elucidated the overlap among different influential factors. Future investigations should address the metabolism of metastatic cancer cells in hydrogels that recapitulate a range of bone stiffnesses. A holistic approach will contribute to understanding how each aspect of the dynamic fertile soil is interwoven to successfully facilitate metastasis.
As prognoses worsen as metastasis increases, further research is needed to understand the niches and create treatments targeted at metastasis. This research is integral to developing therapeutics designed to end metastasis of cancer cells
ACKNOWLEDGEMENTS
I would like to thank Dr. Angela Eeds and Dr. Mention Deweese from the School for Science and Math at Vanderbilt for coordinating my lab research and supporting my science communication; Logan Northcutt for his continuous guidance throughout the research and writing process; and Dr. Marjan Rafat for her recommendations and mentorship.
GLOSSARY
| Mechanotransduction | Mechanical stress exerted upon the cell transforms into chemical signals and an adaptive cell response |
| Hydrogel | 3-Dimensional network of crossed polymer chains used to recapitulate human microenvironments |
| Adipocyte | Fat cell |
| Organotropism | Cancer cells are attracted to particular areas of the body |
| Premetastic Niche | Secondary environment outside of the primary region of cancer that is conducive to housing cancer cells |
| Cortical bone | The dense tissue surrounding the exterior of the bone that coats the spongey, cancellous inside of the bone |
REFERENCES
- Zuo, D. Yang, Q. Yang, H. Tang, Y. Fu, Y. Wan, Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nature. 11, 1578 (2020).
- Haider, D. Smit, H. Taipaleenmaki, The endosteal niche in breast cancer bone metastasis. Frontier Oncology. 10, 335 (2020)
- Guise, The vicious cycle of bone metastases. Journal of Musculoskeletal and Neuronal Interaction. 6, 570-572 (2002)
- Jie, X. Zhang, C. Xu, Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: mechanisms and clinical applications. 46, 845-851 Oncotarget. (2017).
- Gomez, N. Tracey, R. Ma, B. Qian, V. Brunton, Mouse models of metastasis: progress and prospects. Disease Models and Mechanisms. 9, 1061-1074 (2017).
- Valastyan, R. Weinberg, Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2, 275-292 (2011).
- Thery, A. Meddis, L. Cabel, C. Proudhon, A. Latouche, J. Pierga, F. Bidard, Circulating Tumor Cells in Early Breast Cancer. JNCI Cancer Spectrum. 3, pkz026 (2019).
- Eckert, T. Lwin, A. Chang, J. Kim, E. Danis, L. Ohno-Machado, J. Yang, Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 19 372-86 (2011).
- Linder, C. Wiesner, M. Himmel, Degrading devices: invadosomes in proteolytic cell invasion. Annual Review of Cell and Developmental Biology. 27, 185-211 (2011).
- Kühnel, O. Blau, K. Nogai, I. Blau, The Warburg Effect in Multiple Myeloma and its microenvironment. Medical Research Archives. 5, 201-243 (2017)
- Yadav, S. Kumar, T. Marlowe, A. Chaudhary, R. Kumar, J. Wang, J. O’Malley, P. Boland, S. Jayanthi, T. Kumar, N. Yadava, D. Chandra, Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Nature. 6, 1969 (2015).
- Wu, J. Wu, Q. Zhao, S. Fu, J. Jin, Emerging roles of aerobic glycolysis in breast cancer. Clinical and Translational Oncology. 22, 631-646 (2020).
- Shafat, T. Oellerich, S. Mohr, S. Robinson, D. Edwards, C. Marlein, R. Piddock, M. Fenech, L. Zaitseva, A. Abdul-Aziz, J. Turner, J. Watkins, M. Lawes, K. Bowles, S. Rushworth, Leukemic blasts program bone marrow adipocytes to generate a protumoral microenviroment. Blood. 129, 10 (2017).
- Ladanyi, A. Mukherhee, H. Kenny, A. Johnson, A. Mitra, S. Sundaresan, K. Nieman, G. Pascual, S. Benitah, A. Montag, D. Yamada, N. Abumrad, E. Lengyel, Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 37, 2285-2301 (2018).
- Nieman, H. Kenny, C. Penicka, A. Ladanyi, R. Buell-Gutbrod, M. Zillhardt, I. Romero, M. Carey, G. Mills, G. Hotamisligil, D. Yamada, M. Peter, K. Gwin, E. Lengyel, Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine. 17, 1498-1503 (2011).
- Chambers, S. Matosevic, Immunometabolic dysfunction of natural killer cells mediated by the Hypoxia-CD73 Axis in solid tumors. Frontiers in Molecular Biosciences. 6, 10-59(2019).
- Vaupel, K. Schlenger, C. Knoop, M. Höckel, Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Research. 51, 12 (1991).
- Hohenberger, C. Felgner, W. Haensch, P. Schlag, Tumor oxygenation correlates with molecular growth determinants in breast cancer. Breast Cancer Research Treatments. 48, 97-106 (1998).
- Spencer, F. Ferraro, E. Roussakis, A. Klein, J. Wu, J. Runnels, W. Zaher, L. Mortensen, C. Alt, R. Turcotte, R. Yusuf, D. Cote, S. Vinogradov, D. Scadden, C. Lin, Direct measurements of local oxygen concentration in the bone marrow of live animals. Nature. 508, 269-273 (2014).
- Liu, G. Semenza, H. Zhang, Hypoxia-inducible factor 1 and breast cancer metastasis. Zhejian University Science. 16, 32-43 (2015).
- Sawicki, E. Ovadia, L. Pradhan, J. Cowart, K. Ross, C. Wu, A. Kloxin, Tunable synthetic extracellular matrices to investigate breast cancer response to biophysical and biochemical cues. APL Bioengineering. 3, 016101 (2019).
- Chaudhuri, S. Koshy, C. Cunha, J. Shin, C. Verbeke, K. Allison, D. Mooney, Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Materials. 13, 970-978 (2014).
- Northcutt, A. Suarez-Arnedo, M. Rafat, Emerging Biomimetic Materials for Studying Tumor and Immune Cell Behavior. Biomedical Engineering 48, 2064-2077 (2019).
- M. Cavo, M. Caria, I. Pulsoni, F. Beltrame, M. Fato, S. Scaglione, A new cell-laden 3D alginate Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed in vivo. Sci. Rep. 8, 1-12 (2016).
Posted by John Lee on Thursday, May 20, 2021 in May 2021.
Tags: adipocytes, bone metastasis, breast cancer, stiffness