Dye-Sensitized Solar Cells: Optimization of Parameters to Maximize Efficiency
ABSTRACT
Dye-sensitized solar cells are a promising new approach toward sustainable energy production, converting solar energy into electricity through an electrochemistry phenomenon. They show potential as a cost-effective and environmentally friendly alternative to conventional silicon-based photovoltaic cells, as they can be produced from inexpensive, non-toxic materials like titanium dioxide and natural dyes. However, a major challenge limiting their widespread use is their low efficiency, particularly when produced from natural dyes, as compared to traditional solar cells. The objective of this investigation was to optimize each parameter during fabrication to enhance the power output and efficiency of dye-sensitized solar cells. Titanium dioxide nanoparticles were mixed with acid, annealed onto indium tin oxide glass, and bonded with anthocyanin molecules from fruit dyes. The highest recorded average efficiency was 0.0493%, achieved using blackberry dye, 0.1 M hydrochloric acid, and a halogen lamp at 40 V. The highest average power density was 2663 nW/cm2, achieved by the same cells under the halogen lamp at 110 V. These results highlight blackberry as a promising natural dye for increasing efficiency, suggesting the need for further research into anthocyanin types in dye-sensitized solar cells. Additionally, 0.1 M hydrochloric acid was identified as the optimal treatment for titanium dioxide, aligning with existing literature findings. The variations in lighting conditions that produced the highest power density and highest efficiency in cells suggest the presence of a rate-limiting factor, such as electron diffusion through the titanium dioxide layer.
INTRODUCTION.
The dye-sensitized solar cell (DSSC) is a thin-film solar cell that combines solar power with an electrochemistry phenomenon to produce electricity [1]. There is increasing interest in solar energy as an environmentally friendly source of renewable energy [1, 2], but large-scale use of standard silicon-based photovoltaic cells is limited, due to their high cost of creation and maintenance [1]. Dye-sensitized solar cells are a promising alternative, with a low-cost fabrication process and materials [2]. Previous research includes investigations into redox mediators and device structures, development of synthetic sensitizers, and acid pre-absorption onto the titanium dioxide film, to increase DSSC efficiency [3]. The goal of this research was to use natural dyes (from fruits such as blackberries, pomegranates, raspberries, and blueberries), a non-precious metal oxide, and a carbon catalyst to optimize the performance of DSSCs.
A DSSC is composed of a working electrode (WE), an electrolyte acting as redox mediator, and a counter electrode (CE) [4]. The WE is composed of a conductive glass slide coated in a thin film of titanium (IV) dioxide (TiO2) and a dye sensitizer. The CE is composed of another conductive glass slide coated with an electrocatalyst, such as platinum or carbon [5]. The CE functions as an inert electrode, providing a solid substrate for catalyzing the electrolyte electron transfer.
Working Principles.
Figure 1 depicts the working mechanisms of the DSSC connected to a circuit. The DSSC uses an iodine solution containing iodide and triiodide ions (I– and I3–) as redox mediator. As light reaches the WE, the dye molecules absorb the photons and become excited. Electrons of the dye become promoted from the ground state to the excited state. They are transferred into the conduction band of the nanoporous TiO2 through electron injection [5]. TiO2’s conduction band lies below the excited state of the dye in energy [6]. TiO2 has a wide band gap of 3.2 eV [1] and is represented in grey in Figure 1, with anthocyanin molecules attached. The electrons diffuse through the TiO2 nanoparticles. When they reach the conductive oxide glass, they join the circuit and perform work on a load. At the same time, the regeneration of the ground state of the dye occurs when the oxidized dye receives an electron from the iodide ion, which is in turn oxidized to triiodide [5]. The electrons flowing through the circuit arrive at the CE. The triiodide ion receives these electrons and is reduced back to iodide in a reaction catalyzed by the electrocatalyst.
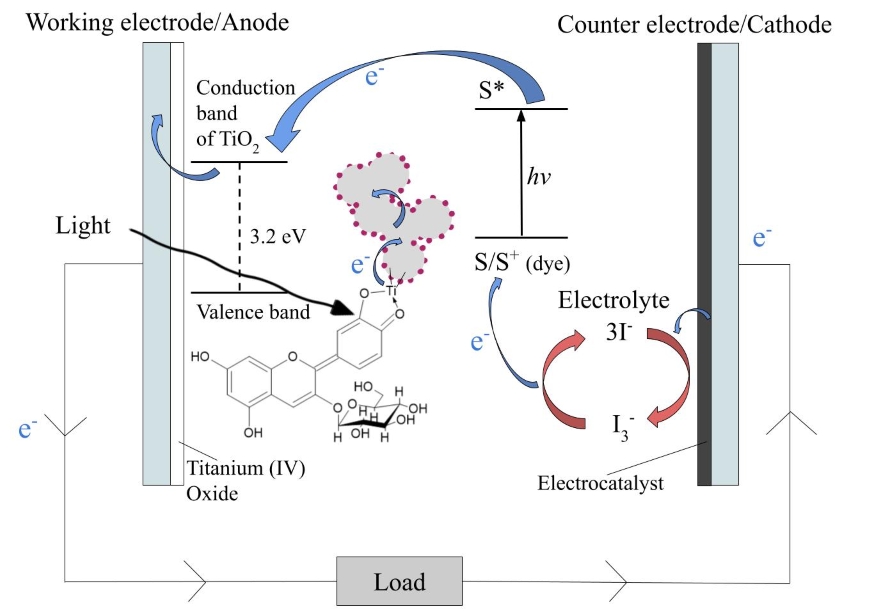
Equations (1) – (4) are the chemical equations for the reactions in the DSSC that allow it to convert light energy into electrical energy [5, 6]. The dye molecules (denoted as S) act as photosensitizers. The electrically excited dye is represented by S*, and by S+ after its oxidization. I– and I3– represent the iodide and triiodide ions respectively.
\[S+h\nu\longrightarrow S^\ast\tag{1}\]
\[S^\ast\longrightarrow S^++e^-\ \left(TiO_2\right)\tag{2}\]
\[I_3^-+2e^-\longrightarrow3I^-\tag{3}\]
\[S^++\frac{3}{2}I^-\longrightarrow S+\frac{1}{2}I_3^-\tag{4}\]
Parameters.
Parameters that may impact the efficiency of the DSSC include anthocyanins in the juice, morphology and acid treatment of the TiO2, and lighting.
Titanium Dioxide.
The TiO2 nanoparticles used in DSSCs are mesoporous. It is hypothesized that reducing the particle size of TiO2 could enhance the cell’s overall efficiency. Because numerous anthocyanin dye molecules are bonded to each nanoparticle, increasing the surface area-to-volume ratio of TiO2, which is achieved by decreasing particle size, would allow for more dye molecules to bond to the surface [7]. An increase in the surface area of TiO2 also results in an increased area for incident photons, and therefore an increase in the incident photon to current efficiency (IPCE).
Anthocyanins & Light.
First, due to its wide band gap, TiO2 absorbs very little visible light [2], resulting in the need for dyes for light absorption. Anthocyanin dyes chelate with TiO2 and facilitate the transfer of injected electrons to its conduction band, allowing the cell to function under visible light [1, 2]. Anthocyanin molecules require carbonyl (=O) or hydroxyl (–OH) groups that can coordinate the bond with Ti4+. Examples include cyanidin (Figure S1) and delphinidin. Fruits rich in these anthocyanins, including pomegranate, blackberry, blueberry, cranberry, and raspberry, can be successfully used in DSSCs.
Second, it is hypothesized that increasing light intensity will improve cell performance. Light intensity can be increased by increasing the voltage of the variable transformer controlling the halogen lamp. As the voltage increases, both the output power density and efficiency are expected to rise, as more photons can be absorbed and excite electrons.
Acid Treatment.
Acid type and concentration may affect structural characteristics of the sintered TiO2, such as porosity and particle size [8]. An increase in porosity will increase the IPCE, so different acid types and concentrations should be tested to optimize acid treatment and maximize DSSCs’ efficiency.
MATERIALS AND METHODS.
Cell Construction.
Indium Tin Oxide (ITO) coated conductive glass of 5 Ω/sq, ≥77% transmittance, dimensions 2.5 x 2.5 cm was used for the cells. TiO2 was mixed with acid in a 1 g to 2 mL ratio with a mortar and pestle for 15 minutes, then doctor-bladed onto the glass with 1 mil polyimide tape, for an active area of 1.5 x 1.5 cm. The coated glass slide was annealed at 400 °C for 10 minutes, then dyed in fruit juice for one hour to produce the WE. The CE was prepared by coating another glass slide with carbon (soot). The final cell was assembled using binder clips, and two drops of 0.05 M I2 and 0.5 M KI in propylene glycol were added as redox electrolyte. The lighting setup consisted of a halogen lamp connected to a variable transformer, illuminating a wooden light box covered in aluminum tape, with the cell placed at the center. A neutral-density (ND) filter was used for some trials. The DSSC was connected to the circuit via alligator clips with aluminum contacts.
Voltage and Current Measurement.
Due to the small magnitude of the current, it could not be measured with a handheld multimeter. Instead, a custom circuit was created to amplify and digitize the voltage and current signal. The schematic is shown in Figure S2. A 10 MΩ potentiometer was used as an adjustable load to probe the entire current-voltage profile of the DSSC.
Autoclaving Titanium Dioxide.
A modified procedure for reducing TiO2 particle size by Panda et al. was used to maximize DSSC efficiency [9]. Three grams of TiO2 powder were mixed with 10 M NaOH (aq) and treated in a hydrothermal autoclave placed in an oven at 150 °C for 24 hours, then washed with 0.1 M HCl (aq) to neutralize [9]. The mixture was then dried on a hotplate for one hour. The resulting TiO2 chunks were grounded with a pestle and mortar for 30 minutes.
The HPC330X spectrometer by HOPOOCOLOUR was used to obtain spectra of the various lighting used.
RESULTS.
Experimental Graphs.
Spectral density-wavelength graphs of various lighting sources used for DSSCs are presented in Figure S3. These lighting sources provided the input power for the DSSCs. Figure S3A shows the graphs created by the halogen lamp at different voltages or with an ND-filter, compared to the spectral-density wavelength graph of daylight at mid-day. Figure S3B shows the graphs created by the green and red lasers, whose spectral densities were significantly higher than those from the halogen lamp. The areas under these spectral density-wavelength graphs were used to determine input power density for efficiency calculations.
Current density-voltage curves (I-V curves) for each DSSC were obtained, with two identical cells created for each set of parameters, labelled as S1 and S2 in the figures. The control parameters included blackberry juice, regular TiO2, 0.1 M HCl (aq), and the halogen lamp at 110 V. Each parameter was varied independently, with the other parameters kept at control.
Autoclaved TiO2.
The I-V curves of cells fabricated with hydrothermally autoclaved TiO2 were compared to cells made with regular TiO2 in Figure S4. The average power densities and percentage efficiencies are presented in Table 1. Cells containing autoclaved TiO2 exhibited significantly lower efficiencies than those with normal TiO2.
| Table 1. Average power density produced and average efficiency of DSSCs created from normal TiO2 compared to those created from hydrothermally autoclaved TiO2. | ||
| Average Power Density (nW/cm2) | Average % Efficiencya | |
| Normal TiO2 | 2663 | 0.008184 |
| Autoclaved TiO2 | 977.7 | 0.003005 |
| a Calculated from the average power density of the cell (output) divided by the power density of the lighting (input), multiplied by 100%. | ||
Acid Treatment.
Figure S5 shows the I-V curves of cells fabricated with hydrochloric and acetic acid, at 0.1 M and 1.0 M concentrations. Table 2 provides their power density and efficiency. Cells made with 0.1 M HCl (aq) achieved the highest efficiency and power density, followed by those made with 1.0 M acetic acid, 0.1 M acetic acid, and 1.0 M HCl.
| Table 2. Average power density and percent efficiency of cells with TiO2 treated with 0.1 M or 1.0 M hydrochloric or acetic acid. | ||
| Acid type and concentration | Average Power Density (nW/cm2) | Average % Efficiency |
| 0.1 M HCl | 2663 | 0.008184 |
| 1.0 M HCl | 108.8 | 0.0003340 |
| 0.1 M Acetic Acid | 208.2 | 0.0006400 |
| 1.0 M Acetic Acid | 1274 | 0.003915 |
Fruit Dye.
Figure S6 presents the I-V curves of cells created from four different fruit juices as dye sensitizers. Table 3 lists their average power densities and efficiencies. Cells dyed with blackberry exhibited the highest efficiency, followed by pomegranate. Raspberry and blueberry cells demonstrated similarly low efficiencies.
| Table 3. Average power density and percent efficiency of DSSCs made with blackberry, pomegranate, blueberry, or raspberry fruit dyes. | ||
| Fruit Dye | Average Power Density (nW/cm2) | Average % Efficiency |
| Blackberry | 2663 | 0.008184 |
| Pomegranate | 2375 | 0.007300 |
| Blueberry | 749.5 | 0.002304 |
| Raspberry | 757.0 | 0.002327 |
Lighting Intensity.
Figure S7 depicts the I-V curves of cells under the halogen lamp at various voltages from the variable transformer. Table 4 depicts the average power density and efficiency for each lighting intensity. The highest average power density was observed at 110 V, followed by 70 V and 40 V. The average percent efficiency exhibited an inverse relationship with voltage; 40 V resulted in the highest efficiency, followed by 70 V, while 110 V exhibited the lowest efficiency.
| Table 4. Average power density and percent efficiency of DSSCs under the halogen lamp at various voltages. | ||
| Halogen Lamp Voltage | Average Power Density (nW/cm2) | Average % Efficiency |
| 110 V | 2663 | 0.008184 |
| 70 V | 1854 | 0.02353 |
| 40 V | 465.0 | 0.04934 |
Lighting Types.
I-V curves of DSSCs under different types of lighting including lasers and an ND filter are shown in Figure S8. Their corresponding average power density and percent efficiency are shown in Table 5. For cells with the ND filter, they were positioned under the filter, within the halogen lamp box at 110 V. The cells illuminated with lasers had the laser directly aimed at them. The highest power density and efficiency were produced by cells under the halogen lamp at 110 V without the filter, followed by cells under the ND filter at maximum transmittance. The next highest average power density was produced by the green laser’s cell, followed by those of the red laser and the ND filter at minimum transmittance. Cells under the filter at minimum transmittance demonstrated the third highest efficiency, followed by the red and green laser-illuminated cells.
| Table 5. Average power density and percent efficiency of DSSCs under various types of lighting. | ||
| Lighting Type | Average Power Density (nW/cm2) | Average % Efficiency |
| Green laser a | 1338 | 0.001380 |
| Red laser | 353.3 | 0.001487 |
| Maximum transmittance 110 V | 1396 | 0.003825 |
| Minimum transmittance 110 V | 45.50 | 0.001658 |
| No ND filter 110 V | 2663 | 0.008184 |
| a Green laser was the lighting source for only one cell, so the values in the average power density and average efficiency column are S1’s power density produced and its efficiency. | ||
DISCUSSION.
Effect of Autoclaving TiO2.
The DSSCs created from TiO2 powder that underwent the autoclaving procedure showed significantly poorer performance compared to those made from untreated TiO2. However, this does not disprove the hypothesis that reducing TiO2 particle size to increase surface area could improve cell efficiency. After autoclaving, the TiO2 particles appeared larger and less powdery than the normal particles, and after acid treatment, the TiO2 film appeared significantly grainier. These observations suggest that the TiO2 participles may have clumped together, reducing their surface area, and resulting in lower power and efficiency for the autoclaved TiO2 cells than the non-autoclaved ones. For future experiments, it is recommended that the TiO2 be dried at 80°C for a full 24 hours and heated at 500°C to follow the full procedure in [9]. Further research to decrease TiO2 particle size and measure effects on efficiency merit exploration.
Acid Treatment Optimization.
DSSCs treated with 0.1 M hydrochloric acid significantly exceeded those treated with acetic acid and other concentrations in efficiency. This aligns with literature findings, where Hao et al., also reported that hydrochloric acid produced higher efficiency cells than sulphuric, nitric, or phosphoric acid [8]. Hao et al. found 0.1 M to be the optimal concentration for hydrochloric acid and suggested HCl to be superior to other acids due to its properties as a strong and non-oxidizing acid. Unlike nitric acid, a strong oxidizing acid, HCl does not destroy the surface structure of TiO2, or introduce impurities to it [8]. Additionally, cells treated with 1.0 M HCl demonstrated lower efficiency than those treated with 0.1 M, likely due to increased aggregation of TiO2 particles and larger particle sizes when the concentration of HCl is increased beyond 0.1 M, resulting in a decline in the absorption of dye molecules [8].
Although sulphuric and phosphoric acid are both non-oxidizing, the sulfate (SO42-) and phosphate (PO43-) ions can bond with the oxygen ion (O–) at the surface of TiO2, blocking the absorption of dye molecules [8]. This is due to the bond between the oxygen ion and the sulphate or phosphate ion being stronger than the bond between the oxygen ion and the carboxyl (-COOH) [8]. However, this issue is less likely to be present with acetic acid (CH3COOH (aq)), which also contains the carboxyl group. Acetic acid also cannot be easily oxidized, but is a weak acid, unlike HCl (aq) and H2SO4 (aq). Therefore, recommendations for future research include further comparisons of acetic acid with various other acids to optimize DSSC efficiency. Bang et al. investigated volumes of acetic acid (1-30 mL) for DSSCs and found that generally higher volumes resulted in slightly improved efficiencies [10]. This is consistent with the results of this research, as 1.0 M acetic acid yielded significantly better performance than 0.1 M. It is suggested that this may be because an increase in acetic acid in the TiO2 paste increases the porosity of the layers of TiO2, resulting in greater surface area for dye absorption [10].
Fruit Dye Optimization.
This research’s results on efficiency align with findings from literature. Ghann et al. reported that cells dyed with pomegranate achieved the highest efficiency cell at 2.0%, followed by blackberry at 1.4%, cranberry at 1.2%, and blueberry at 0.2% [2]. Consistent with their findings, this study found that blueberry yielded the lowest efficiency. Furthermore, blackberry and pomegranate were found to be the two most effective fruits for optimizing efficiency, although the precise ranking differed. While Ghann et al. noted that pomegranate dye maximized efficiency, followed by blackberry, results from this study indicated that blackberry outperformed pomegranate. This slight discrepancy may stem from differences in the specific varieties of pomegranates used. Pomegranates vary considerably in their juice colour, and the pomegranate juice used for DSSCs in this research appeared to be on the lighter end of the spectrum. This observation suggests a potentially lower quantity of anthocyanins, contributing to a lower efficiency. In contrast, the pomegranates used by Ghann et al. may be darker in colour and contain higher amounts or different types of anthocyanins, with greater capacities for light absorption or bonding with TiO2, thereby resulting in higher efficiencies that surpassed those achieved with blackberry.
For future DSSC production, it is recommended that anthocyanins be extracted from the fruits and separated by type to investigate the effectiveness of each type of anthocyanin. This approach would also bypass issues related to variations in anthocyanin concentrations and types within the same fruit variety.
Lighting Sources.
First, the results confirm the prediction that increasing lighting intensity enhances the power output of DSSCs. Cells exposed to higher voltages of the halogen lamp (40 V, 70 V, and 110 V in increasing order of power density) produced greater power, as expected, due to the increased photon flux hitting the active area. However, the cells’ efficiency followed the reverse trend, with the highest light intensity (110 V) resulting in the lowest efficiency. This discrepancy occurred because efficiency accounts for input power, which increases as light intensity increases. Although higher voltage lighting produces greater power density, there is a trade-off such that the substantial increase in input power reduces overall efficiency. These results suggest the presence of one or more rate-limiting components in the cells, such as the redox reaction, or electron diffusion through the TiO2 layer.
Second, cells exposed to the green laser lighting generated significantly more power than those under the red laser, seeming to indicate better absorption of green wavelengths than red for DSSCs. However, cells under the red laser exhibited slightly higher efficiency than those under the green laser. This discrepancy is due to the green laser’s much higher intensity, as seen in the spectral density graph (Figure S3B), which results in a much greater input power, while the power output by the cell under the green laser is not as much greater than under the red laser. To certify that DSSCs perform better under green light due to better absorption, future experiments should use different light sources with similar intensity, to have similar input power, perhaps through the use of white lighting and filters.
Lastly, Table 5 further compares the performance of cells under an ND filter at maximum and minimum transmittance. It shows that while the average power density at maximum transmittance is approximately 30 times greater than at minimum transmittance, the efficiency is only about twice as high. This is due to the input power being much greater at minimum transmittance, once again pointing to the possible presence of a rate-limiting component in the cells.
CONCLUSION.
Dye-sensitized solar cells generate electricity using solar power and an electrochemistry phenomenon. To make them in this study, titanium dioxide nanoparticles were mixed with acid, annealed onto ITO glass, and bonded with anthocyanin molecules from fruit dyes. This research found specific optimization of parameters to maximize DSSC efficiency. The acids tested were hydrochloric acid and acetic acid of 1.0 M and 0.1 M. The fruit dyes were blackberry, pomegranate, raspberry, and blueberry. The lighting sources were a halogen lamp at 110 V, 70 V, and 40 V, an ND filter, and red and green lasers. The highest average efficiency achieved was 0.0493%, using blackberry dye, 0.1 M hydrochloric acid, and the halogen lamp at 40 V. The highest average power density was 2663 nW/cm2, obtained from the same cells under 110 V lighting. The results of this study align with certain previous findings in literature. Further studies on specific anthocyanin types and potential rate-limiting factors affecting DSSC performance are recommended. The findings contribute to the body of research on DSSCs by highlighting specific parameters that improve the overall power density and efficiency of DSSCs. Through selecting suitable fruit dyes and optimizing acid concentrations, this study further helps guide research in low-cost, natural, and environmentally friendly alternatives to both traditional silicon-based solar cells and synthetic dyes in DSSC fabrication.
ACKNOWLEDGMENTS.
I would like to express my sincere thanks to Jim Chen for his invaluable guidance and support throughout my research, and for providing essential lab space and materials. I am especially grateful for his work in creating the custom circuit and lighting setup, and for his insightful feedback on this manuscript.
SUPPORTING INFORMATION.
The following figures can be found in the Supporting Information document:
- Cyanidin-3-glucoside molecule
- Diagram of the circuit used for current and voltage measurements
- Spectral density spectra of the different lightings used
- I-V of cells with varying parameters
REFERENCES
- B. O’Regan, M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 353, 737–740 (1991).
- W. Ghann, H. Kang, T. Sheikh, S. Yadav, T. Chavez-Gil, F. Nesbitt, J. Uddin, Fabrication, Optimization and Characterization of Natural Dye Sensitized Solar Cell. Scientific Reports. 7 (2017).
- Y. Ren, D. Zhang, J. Suo, Y. Cao, F. T. Eickemeyer, N. Vlachopoulos, S. M. Zakeeruddin, A. Hagfeldt, M. Grätzel, Hydroxamic acid preadsorption raises efficiency of cosensitized solar cells. Nature. 613, 60–65 (2022).
- D. Ghernaout, A. Boudjemline, N. Elboughdiri, Electrochemical engineering in the core of the dye-sensitized solar sells (DSSCs). Open Access Library Journal. 7, 1–12 (2020).
- G. P. Smestad, M. Gratzel, Demonstrating Electron Transfer and Nanotechnology: A Natural Dye-Sensitized Nanocrystalline Energy Converter. Journal of Chemical Education. 75, 752–756 (1998).
- K. Sharma, V. Sharma, S. S. Sharma, Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Research Letters. 13 (2018).
- L. A. Dobrzański, M. M. Szindler, M. Szindler, K. Lukaszkowicz, A. Drygała, M. Prokopiuk vel Prokopowicz, Nanocrystalline TiO2 Powder Prepared by Sol-Gel Method for Dye-Sensitized Solar Cells. Archives of Metallurgy and Materials. 61, 833–836 (2016).
- S. Hao, J. Wu, L. Fan, Y. Huang, J. Lin, Y. Wei, The influence of acid treatment of TiO2 porous film electrode on photoelectric performance of dye-sensitized solar cell. Solar Energy. 76, 745–750 (2004).
- J. Panda, U. P. Singh, R. Sahu, Synthesis, characterization of TiO2 nano particles for enhancement of electron transport application in DSSC with Cu-BPCA Dye. IOP Conference Series: Materials Science and Engineering. 410 (2018).
- H.-G. Bang, J.-K. Chung, R.-Y. Jung, S.-Y. Park, Effect of acetic acid in TiO2 paste on the performance of dye-sensitized solar cells. Ceramics International. 38, S511–S515 (2012).
Posted by buchanle on Tuesday, June 24, 2025 in May 2025.
Tags: Anthocyanins, Dye-Sensitized Solar Cells, Efficiency, Solar Energy, Titanium Dioxide

