A Microfluidic Blood Brain Barrier Chip Model for Studying the Effects of VEGF on Brain Endothelial Cells (HCMEC/D3)
ABSTRACT
The blood vessels in the brain have a protective barrier that prevents harmful pathogens from reaching the brain. It is believed that Type 2 Diabetes(T2D) might play a role in weakening this barrier, leading to plaques of protein building up in the brain, which is a hallmark of Alzheimer’s disease and related dementias (ADRD). The main purpose of this study is to develop a tri-channeled, “organ on a chip” model that mimics the protective barrier and to stimulate diabetic conditions to investigate how T2D might influence ADRD development. We tested the effect of a protein called VEGF, which helps form blood vessels, on brain endothelial cells(cells that make up the protective barrier) to see if we were able to induce tube-like structures within the chip. We found VEGF did induce a significant amount of tube-like formation within the right channel of the chip. Shockingly, patients diagnosed with T2D have a 50% higher risk of developing ADRD. This is alarming because 1 out of 10 Americans have T2D and are therefore at risk for developing ADRD. Thus, this research has a high potential to improve understanding of T2D’s effect on ADRD, aiding future treatment methods.
INTRODUCTION.
The human brain uses an intricate network of blood vessels to maintain homeostasis. Within these blood vessels, a barrier, between the blood within the vessels and the brain tissue, is formed to protect against harmful pathogens and infection. This barrier is known as the Blood-Brain Barrier (BBB), a selectively permeable membrane composed of microvascular endothelial cells, astrocytes, pericytes, and other supporting neural cells [1].
Alzheimer’s disease and related dementias (ADRD) have been linked to BBB dysfunction along with many other neurological disorders [2]. Vascular Endothelial Growth Factor (VEGF) is a crucial protein involved in the formation and maintenance of blood vessels. Furthermore, VEGF plays an important role in angiogenesis: the creation of new blood vessels from old or damaged vasculature [3]. Therefore, within the BBB, VEGF can influence the permeability and structural integrity of the barrier [4]. Additionally, Type 2 Diabetes (T2D) is a metabolic disease that affects 1 in 10 Americans and increases the risk for ADRD by almost 50% [5], [6], [7]. Finding the relationship between these two diseases is imperative to preventing a large percentage of the population from developing ADRD. This relationship is believed to be caused by BBB dysfunction, leading to an increase of amyloid-β (Aβ) plaques within the brain. These plaques are a common aspect among people diagnosed with ADRD [8], [9], [10]. Thus, dysfunction of the BBB could play a critical role in the development of ADRD [11].
Advanced in vitro models that replicate the human BBB environment are essential to studying these complex interactions [12]. Traditional models, such as transwell assays, are insufficient at mimicking the BBB’s intricate microenvironment and cellular interactions [11]. Microfluidic technology, however, offers a promising alternative by providing precise control over the cellular and extracellular environment. This technology also allows for the creation of more physiologically relevant models. Our research aims to develop a 3D microfluidic BBB model that closely mimics the physiological conditions of T2D patients to study the effects of T2D on Aβ accumulation and its impact on ADRD development. We designed a microfluidic chip with three channels: a central channel filled with hydrogel and two side channels filled with endothelial cell media. By introducing VEGF, we aim to promote the formation of tube-like structures that mimic brain capillaries within the chip. Initial experiments compare chips with and without VEGF to observe differences in vascular density and cell viability. Our findings will enhance our understanding of the pathological link between T2D and ADRD, potentially leading to new therapeutic strategies and improving health outcomes for millions of individuals at risk by creating more accurate and reliable preclinical models.
MATERIALS AND METHODS.
Designing and Creating the Microfluidic Chip.
The microfluidic chip had a three-channel design. This included a central channel to house the GelCad hydrogel and two side channels for diffusing endothelial media. All channels were made to be 1 mm wide and had a total volume of 10 milliliters each. Within the central channel, two opposing protrusions were added to promote sheer stress and increase cell vitality[13]. A negative mold of the chip was designed using Fusion 360 and was printed with a Formlabs Form 3 resin printer. Once the printing was complete, the resin mold was coated with a thin layer of Parylene to prevent the sticking of polydimethylsiloxane (PDMS) in later processes. After, PDMS, made with a 10:1 ratio of Sylgard 184 silicone base to curing agent, was poured into the mold and left to sit in a vacuum chamber for 15-30 minutes to remove air bubbles. Lastly, the PDMS was cured in an oven at 60°C for 24 hours and then placed into an autoclave to be sterilized for 12 hours (Figure 1).
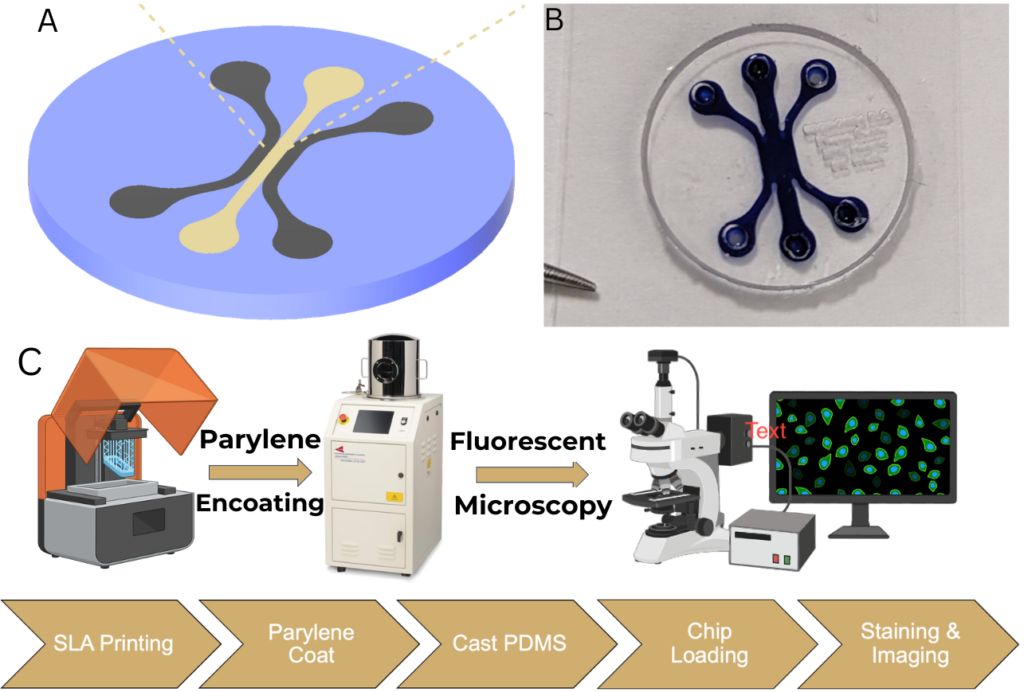
Cell Culture and Hydrogel Preparation.
SCC066 Sigma Aldrich HCMEC/D3 (human cerebral microvascular endothelial cells) were cultured in a Promo Cell C-22110 endothelial cell growth medium at 37°C in a 5% CO2 incubator. The cells were passaged at 80% confluence and utilized between passages 7 and 9. The GelCad hydrogel was prepared in a large batch and was sterilized in an autoclave. After sterilization, the endothelial cells were added to the hydrogel and the endothelial cell media before the chip was loaded.
Experimental Conditions.
We tested six experimental conditions, three of which were “control” while the other three were “VEGF” In the VEGF conditions, 50 ng/mL of Vascular Endothelial Growth Factor (VEGF) was added to the endothelial cell media and hydrogel. In the control conditions, only the media and hydrogel were injected into the chip, with no other additives. Therefore, giving six conditions: Control Central Channel, Control Right Channel, Control Left Channel, VEGF Central Channel, VEGF Right Channel, and VEGF Left Channel.
Loading the Microfluidic Chip.
To load the microfluidic chip, 10 mL of sterilized Gel Cad hydrogel was injected into the central channel with microbial transglutaminase (MTG), made in a batch in house, to crosslink the hydrogel overnight. Once crosslinking had occurred, 10 mL of Promo Cell C-22110 endothelial cell growth medium was injected into each of the two side channels. The chips were then incubated at 37°C in a 5% CO2 atmosphere for 3 days, changing the endothelial cell media daily.
Staining and Imaging.
Once the incubation period had ended, the cells were fixed with 4% paraformaldehyde 158127 Sigma Aldrich) or 60 minutes. Then, the cells were rinsed with endothelial cell media 3 times, with 5 minutes between each rinse. Next, the cells were treated with 0.3% Titron X-100 for 60 minutes and then rinsed again following the same rinsing procedure. Finally, the cells were stained for DAPI, Phalloidin, and anti-ZO-1 antibody for easier visibility of tube-like formation. These stains were used to visualize the cell nuclei, actin filaments (to show the cellular cytoskeleton), and tight junction proteins. The images were taken on a confocal microscope with five images taken per channel.
Data Analysis.
The quantification for vascular density was the proportion of vessel area over the total area of the image. ImageJ was used to automate this quantification over all images taken. Once the data collection had been completed, Excel was used to run a one-tailed homoscedastic T-test between each condition and its corresponding condition (Three T-tests total). For example, one of the T-Tests was between the Control Right Channel and the VEGF Right Channel.
RESULTS.
Qualitative Analysis of Channel Staining.
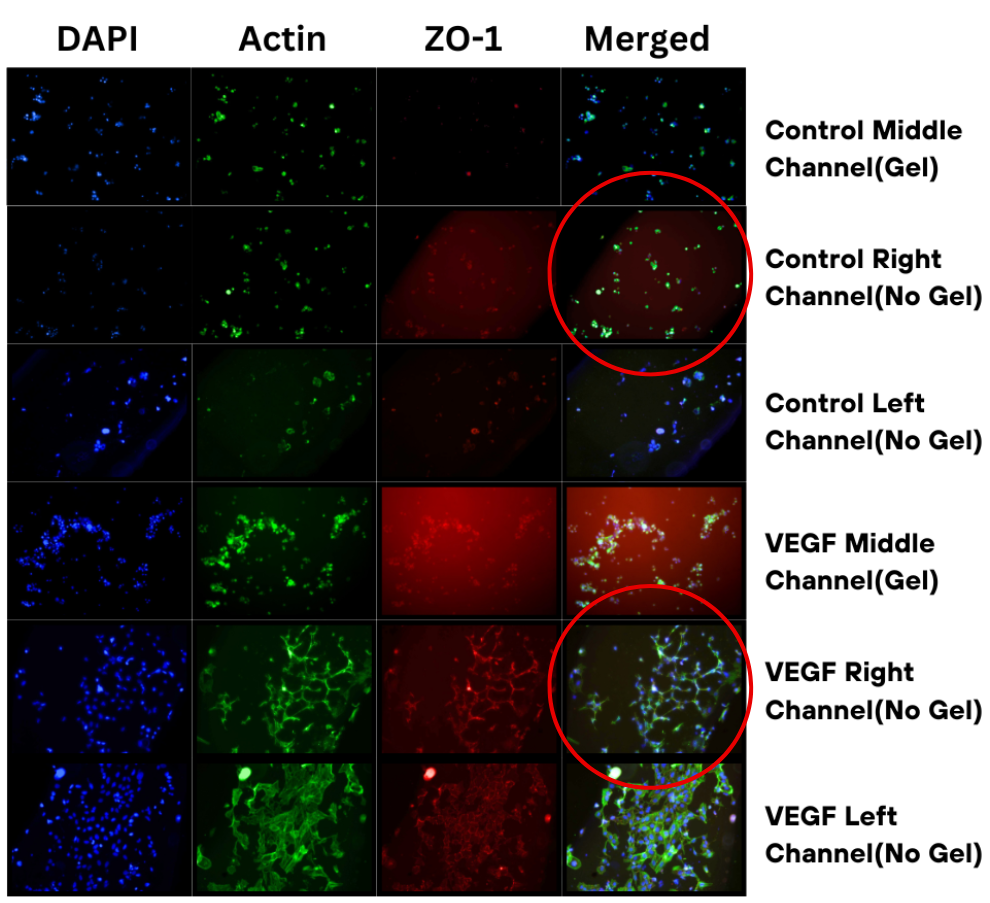
As shown in Figure 2, there was a distinct difference in the cell structure formations between each condition. Within the VEGF-treated channels, the endothelial cells can be seen forming tube-like structures, highlighted by ZO-1(tight junction protein) stains having continuous and defined borders. This tube-like formation can especially be seen within the side channels, containing only media, and has much less of an effect with the central, hydrogel channel. In contrast, the control channels have endothelial cells sparsely separated, forming clusters with no interconnections. There is a lack of tight junction formation, indicated by weak ZO-1 staining, and a meager cytoskeleton organization within the control channels.
Vascular Density (No flow present).
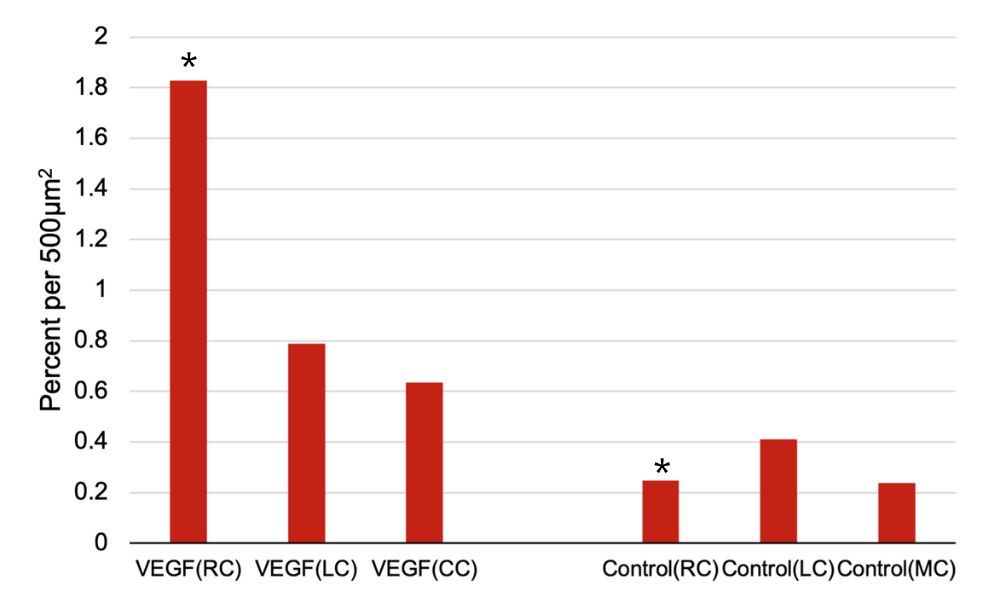
The vascular density was significantly higher in each of the VEGF conditions when compared to their corresponding control condition. Quantitative analysis, via a one-tailed homoscedastic T-test, revealed that the average vascular density in the VEGF Right Channel was 1.82% per 500µm², compared to 0.27% per 500µm² in the Control Right Channel (p<0.05). Similar trends were observed in the Left and Central Channels. (Figure 3) In the VEGF conditions, HCMEC/D3 cells formed distinct tube-like structures, whereas, in the control conditions, cells remained more dispersed with fewer structures. The number of tube-like structures was significantly higher in the average of the VEGF Channels (1.06 structures/mm²) compared to the average of the Control Channels (0.33 structures/mm²) (p<0.05) (Figure 3).
Vascular Density (Flow Present).
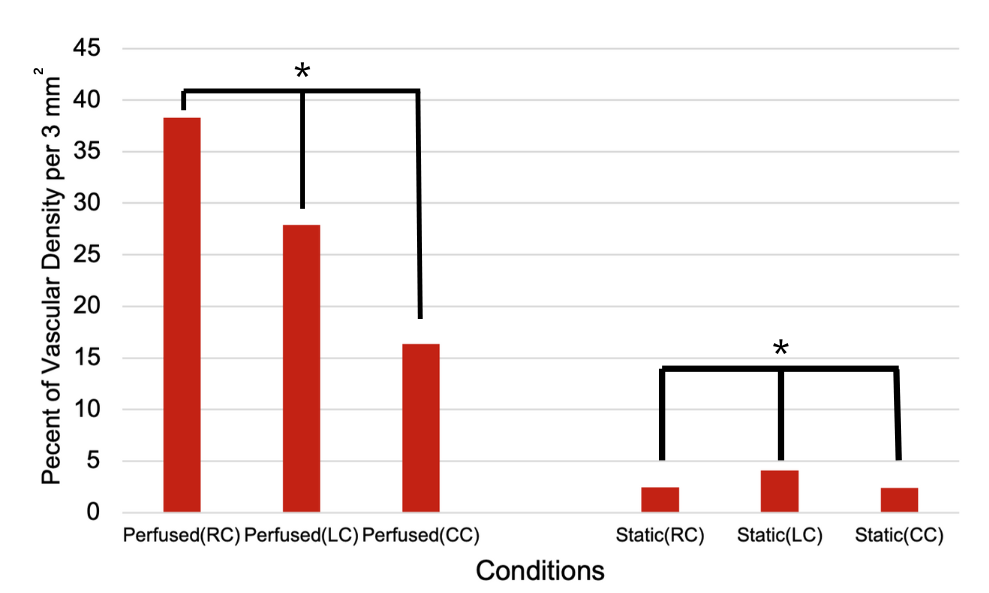
The vascular density was significantly higher in the perfused conditions compared to the static conditions. This is likely due to VEGF being able to penetrate more successfully into the hydrogel and spread the cells out evenly throughout the chip. The perfused condition is much more similar to the dynamic conditions of blood flow in the BBB which could explain why the perfused channels had a more significant vascular density. Quantitative analysis revealed that in the Perfused Right Channel (RC), the vascular density was approximately 40% per 3 mm², while the Static Right Channel showed a vascular density of less than 5% per 3 mm². A similar trend was observed in the Left Channel (LC) and Central Channel (CC), with the Perfused LC and CC demonstrating vascular densities of around 30% and 20% per 3 mm², respectively, compared to the static conditions, which remained low across all channels.
Statistical significance was observed in these differences, as indicated by the asterisks in Figure 4 (p<0.01). These results demonstrate that perfusion significantly enhances tube-like formation within the microfluidic chip, while static conditions result in minimal tube-like development.
DISCUSSION.
The aim of this study was to develop a more physiologically relevant in vitro model of the BBB to study the effects of T2D on Aβ accumulation and its impact on ADRD development. Using VEGF, we successfully induced HCMEC/D3 cells to form tube-like structures within a microfluidic chip that mimics the architecture of brain capillaries. Our results demonstrated a significant difference in vascular density between the VEGF-treated and control conditions, supporting our hypothesis that VEGF could potentially promote angiogenesis in this microenvironment.
However, the unexpected localization of most tube-like structures in the side channels rather than the main hydrogel channel highlights a limitation in our current experimental setup. This phenomenon could be attributed to the concentration gradient of VEGF and the spatial distribution of the endothelial cells. VEGF likely diffused more effectively into the side channels, where the endothelial cells preferentially migrated and formed structures. This suggests that the concentration of VEGF and its delivery method within the chip needs to be optimized to achieve a more uniform vascular response within the channels. Still, these findings are significant because they show that the model can be advanced to the next developmental stage to test its viability and to investigate if it can truly model ADRD development within T2D conditions.
Looking forward, several modifications are planned to enhance the model’s physiological relevance. Increasing the concentration of VEGF tenfold in the main channel and eliminating it from the side channels should help guide endothelial cells to form a more centralized and uniform monolayer, better mimicking the in vivo environment of the BBB. Additionally, the inclusion of a brain compartment within the microfluidic chip, comprising neurons and astrocytes, will be critical for studying the cellular interactions involved in ADRD. These cell types play a significant role in neuroinflammation and Aβ processing, and their incorporation will provide a more comprehensive model for investigating T2D’s effects on the brain.[10]
Furthermore, the role of blood flow in BBB function is very important. Physiological shear stress is known to influence endothelial cell behavior and barrier properties [11]. Thus, future work will involve the integration of a custom-made peristaltic pump to simulate blood flow within the microfluidic chip. The peristaltic pump, consisting of a rotating disc that pinches the tubing to move fluid, will generate flow within the chip, mimicking the hemodynamic forces present in vivo. This differs from the current gravity flow system because the amount of liquid flowing can be controlled. This addition will help in studying the dynamic aspects of the BBB under diabetic conditions and their impact on Aβ transport and accumulation. In conclusion, while our initial results are promising, indicating that VEGF can promote tube-like structures within a microfluidic BBB model, further optimization and incorporation of additional physiological factors are necessary. By refining the VEGF delivery, adding relevant brain cell types, and incorporating flow, we aim to develop a robust and reliable in vitro model that closely replicates human BBB conditions.
ACKNOWLEDGMENTS.
I would like to thank my instructors from the School of Science and Math (SSMV). I would also like to acknowledge the entire Lippmann Lab for their support and lending me the resources to complete my research.
REFERENCES.
- R. Daneman, A. Prat, The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
- Sweeney, M., Sagare, A. & Zlokovic, B. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol14, 133–150 (2018). https://doi.org/10.1038/nrneurol.2017.188
- G. Neufeld, T. Cohen, S. Gengrinovitch, Z. Poltorak, Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9-22 (1999).
- Z. G. Zhang et al., VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 106, 829-838 (2000).
- “Type 2 Diabetes, Cognition, and Dementia in Older Adults: Toward a Precision Health Approach.” Diabetes Spectrum. Accessed Jul. 18, 2024.[Online]Available:https://diabetesjournals.org/spectrum/article/29/4/210/32781/Type-2-Diabetes-Cognition-and-Dementia-in-Older
- “2023 Annual Report | AHR.” Accessed Jul. 18, 2024. [Online]. Available: https://www.americashealthrankings.org/learn/reports/2023-annual-report
- S. Chatterjee, K. Khunti, M. J. Davies, Type 2 diabetes. The Lancet 389, 2239-2251 (2017).
- R. D. Bell, B. V. Zlokovic, Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 118, 103-113 (2009).
- B. V. Zlokovic, Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 28, 202-208 (2005).
- G. Chen et al., Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 38, 1205-1235 (2017).
- M. Campisi, Y. Shin, T. Osaki, C. Hajal, V. Chiono, R. D. Kamm, 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117-129 (2018).
- S. Musafargani et al., Blood brain barrier: A tissue engineered microfluidic chip. J. Neurosci. Methods 331, 108525 (2020).
- P. Vulto, S. Podszun, P. Meyer, C. Hermann, A. Manz, G. A. Urban, Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab. Chip 11, 1596-1602 (2011).
Posted by buchanle on Thursday, June 19, 2025 in May 2025.
Tags: Alzheimer’s, brain, Cells, diabetes, microfluidic, VEGF

