The Design and Modification of an Innovative Loofah Sponge Device for the Removal of Aquatic Microplastics
ABSTRACT
Microplastics, which are sesame seed-sized particles from sources such as cosmetics, synthetic fabrics and tires, are the most common type of ocean debris. Past research into ways of removing microplastics or preventing them from entering the ocean has been unsuccessful in both effectiveness and applicability to real life. In this experiment, natural materials were used to make a device that would be able to remove microplastics from both controlled and natural environments. This device was made using a loofah, which was chosen for its porous quality, stuffed with hair, for its ability to trap and entangle microplastics. Oil, which could increase hydrophobicity, was used to cover the hair in some trials. In all experiments performed, the device was successful, removing on average 64% of microplastics in controlled environments and cleaning various microplastics from natural environments.
INTRODUCTION.
As of 2019, in just one cubic meter of water in the ocean, there are about 8.3 million pieces of microplastics, making them the most common type of ocean debris. These sesame seed-sized particles come from a variety of sources, including cosmetics, synthetic fabrics and tires, and because they are difficult to remove through wastewater treatment facilities, they end up in the ocean and near shorelines [1, 2]. They are harmful to marine life and animals and can harm humans when moved up the food chain [3].
While research into ways of removing or preventing microplastics has been conducted, solutions have not succeeded in both effectiveness and applicability. Timmerman and Velders’ project that used valves to catch microplastics resulted in less than 15% captured [4]. For-sale devices (Cora Ball, Guppyfriend, FibreFree) that capture microfibers within laundry machines rely on people buying and using them routinely and also vary greatly in effectiveness. Other methods, such as Ferreira’s chemical method of using magnets and ferrofluids to effectively remove microplastics, have yet to be used for real-life applications and are difficult to reuse [5].
This work focuses on the idea to incorporate natural materials to make an effective and applicable method of cleaning up microplastics. Hair was chosen due to its ability to trap microplastics and its hydrophobic properties, letting nonpolar microplastic pieces “stick” to hair surfaces [6]. It was put through a preliminary experiment and results confirmed its benefit. The loofah plays a similar role: its porous surfaces and ability to become hydrophobic increase the chances that microplastics will stay inside [7, 8].
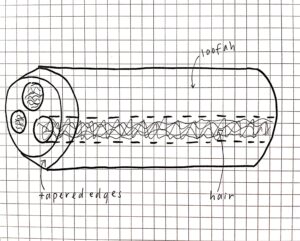
The design of this loofah device needed to account for natural obstacles in the ocean. Because of this, the edges of the loofah have been tapered to prevent other debris from clogging entrances. The amount of hair was also considered as too much means fewer holes for microplastics and too little risks the microplastics floating away. The design shown in Figure 1 was chosen.
Figure 1. Final design of the loofah device, which accounts for obstacles and debris.
MATERIALS AND METHODS.
The loofah device was tested in a three-part experiment: first without oil, then with oil, and finally in a field test.
The first two parts tested its capabilities of cleaning up four types of plastics, namely high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP) and polystyrene (PS). The first part, the “no-oil” group, utilized only the properties of the loofah and the hair. In the second part, the “with-oil” group, the hair was coated with vegetable oil, a nonpolar substance that would help microplastics stick to the hair and loofah, as microplastics are also nonpolar.
The third part of the experiment was a field test. A raft made with three devices was brought to a lake and ran through the water; samples of microplastics from the water stuck in the device will later be photographed.
Controlled Experiments.
The microplastics used in the first two parts of the experiment came from grinding plastic items commonly found in households into small pieces; the HDPE from a flimsy plastic bag, the LDPE from a stiffer plastic bag, the PP from a yogurt container and the PS from an ice cream container. Each plastic will be tested separately.
To make the device, 3.0 g of hair was used and put into the holes of the loofah; for the with-oil group, 0.6 g of vegetable oil was used to coat the hair.
The device was then put into a storage container filled with 75 L of water and 1.0 g of the microplastic being tested; a wavemaker that would simulate the currents in the ocean was also installed. Each trial lasted 30 min and a drop of soap was added to reduce surface tension. Each group was experimented with 4 times. To measure the amount of microplastics removed, the water in the storage container was poured through a sieve with coffee filters, catching the microplastics left in the water, which were then dried and weighed with a scale.
Field Testing.
For the field test, three loofah devices without oil were tied together using cotton string. It was brought to Lake Elizabeth (Fremont, CA) and actively ran through the water by pulling the cotton string attached to it from side to side for 30 minutes. Samples were taken from the loofah device by cleaning out microplastics and other debris trapped inside and straining with a sieve and coffee filters; they were studied under a microscope, and debris identified as microplastics using a squeeze test (squeezing with tweezers as plastics are easier to squeeze, but do not stay bent) were photographed [9].
RESULTS.
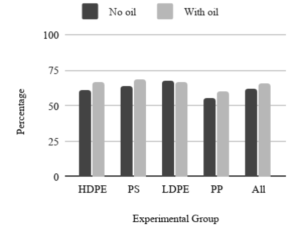
On average, the no-oil experimental groups resulted in 0.62 g out of the added 1.00 g of microplastics being removed (62%) while the second experimental group with oil coated hair resulted in 0.66 g out of the added 1.00 g of microplastics being removed (66%). Figure 2 compares the average percent cleaned for each microplastic group. It can be noted that the data were mostly consistent with little variance and the difference between the trials without oil and the ones with oil resulted in similar percentages, as shown by the last two bars of Figure 2. The loofah device also consistently removed plastics of different types, with similar amounts of microplastics removed for each.
Figure 2. Average % Removed for All Plastic Types. Results from each experimental group comparing plastic type and no-oil and with-oil groups.
Table 1 features the standard deviation for each experimental group separated by the no oil and with oil groups, where N=4 and the averages are of the percent microplastics removed. These numbers show the consistency of data from the experiments.
Table 1. Overview of results from each experimental group with standard deviations to show the consistency of data from the experiments.
| Group | # of Trials (N) | Average (x̄) |
Standard Deviation (σ) |
| HDPE, no oil | 4 | 60.5% | 7.90% |
| HDPE, with oil | 4 | 66.8% | 8.62% |
| PS, no oil | 4 | 63.5% | 21.64% |
| PS, with oil | 4 | 68.5% | 7.00% |
| LDPE, no oil | 4 | 68.3% | 12.61% |
| LDPE, with oil | 4 | 67.3% | 9.54% |
| PP, no oil | 4 | 54.5% | 7.19% |
| PP, with oil | 4 | 60.0% | 17.64% |
Figure 3 is a compilation of photos of microplastics from the 30-minute field testing taken under a microscope. A and B show pieces of microplastic and C shows a strand of microfiber.
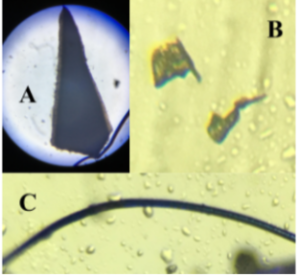 Figure 3. Microplastics from Field Testing at Lake Elizabeth. Various microplastics and a microfiber found inside the loofah after a 30-minute field test.
Figure 3. Microplastics from Field Testing at Lake Elizabeth. Various microplastics and a microfiber found inside the loofah after a 30-minute field test.
DISCUSSION.
The loofah device successfully reduced the amount of microplastics in both controlled and natural environments, with an average of 64% of microplastics removed across controlled experiments. This is due to the porous quality of the loofah and the tangles within the hair, which effectively trap microplastics.
Part two of the experiment, where the hair was coated with oil, did yield a higher success rate; however, the negligible difference (4%) is insignificant and the coat of oil on the hair is unneeded. This likely happened because the oil only helps microplastics stick to hair surfaces, which is unneeded as the microplastics are already entangled within the hair and stuck in the loofah’s pores.
The field testing showed that the loofah device does have the capability of cleaning up microplastics in natural environments and can be effective in ocean microplastic cleanup, as it is easily used and does not trap other organisms. It is able to remove microplastics of different shapes as well, as seen by the larger microplastics and the slimmer microfiber. These devices are also reusable, as the hair is easily taken out and microplastics within the pores washed out.
In the future, experimentation with making the loofah device more applicable in natural environments as well as a more accurate identification or analysis of microplastics, such as using Raman spectroscopy, can be performed.
ACKNOWLEDGMENTS.
Thanks to Dr. Anna Beck and Dr. Irene Liu for their guidance and feedback on this project.
REFERENCES.
- Wood, “We underestimated the amount of microplastic in the ocean – by a lot” (World Economic Forum, 2019).
- NOAA, “What are microplastics?” (National Ocean Service, 2020).
- Toxtown, “Microplastics: your environment, your health” (National Library of Medicine, 2017).
- P. Timmerman, T.D. Velders, “Eliminating microplastics from bodies of water by using an innovative system” (Watertank, 2019).
- Ferreira, “An investigation into the removal of microplastics from water using ferrofluids” (2018).
- Baghdadli et al. A closer look at the complex hydrophilic/hydrophobic interactions forces at the human hair surface. J. Phys.: Conf. Ser. 100 052034 (2008).
- S.A. Cortez, B.I. Kharisov, T.E.S. Quezada et al. Micro- and nanoporous materials capable of absorbing solvents and oils reversibly: the state of the art. Pet. Sci. 14, 84–104 (2017).
- Chen et al. In-depth analysis of the structure and properties of two varieties of natural luffa sponge fibers. Materials 10, 479 (2017).
- Sartain et al. Microplastics: sampling and processing guidebook. Mississippi St. Univ. MASGP-17-069, P3243. (2018).
Posted by John Lee on Wednesday, May 19, 2021 in May 2021.
Tags: Environmental Science, Hair, Loofah, Microplastics