The Creation of a Passive Portable Solar Still: Utilizing Membrane Distillation
ABSTRACT
Across the world today there are many nations that struggle with water sustainability, anywhere from sub-Saharan Africa, to western Europe. In this study, the central aim is to address this issue in less developed areas and create a small-scale solution to provide individual households with fresh water. In the design process, as accessibility is crucial, a small scale solution was used this in turn resulted in the new system being completely passive and compact in order to allow for convenience and small-scale operation. This system encompasses a three-layer membrane design that utilizes membrane distillation. Specifically, a top hydrophilic layer responsible for water absorption, a second hydrophobic layer responsible for water evaporation, and a bottom hydrophilic layer responsible for water condensation. This system was tested in both a membrane distillation (MD) powered system as a controlto ensure the membrane structural integrity and functionality, and a bench-top test demonstrating its passive abilities. The membrane performed well in both tests and demonstrated a promising future for membrane distillation in solving issues such as the world water crisis in an affordable and accessible way.
INTRODUCTION.
Currently four billion people in the world face severe water scarcity for at least one month out of the year, and half a billion face water scarcity year round [1]. The nations that face this problem are not solely located in places such as sub-Saharan Africa, this problem spans the globe from the United States of America all the way to areas in south eastern Asia and Oceania; however, some of these nations, such as the United States and other more developed countries (MDCs), are more fortunate than others, with access to greater fiscal means and more developed infrastructure [2-4].
In many instances, nations that have been classified as having a “water scarcity” do not have access to bodies of fresh water, however, they do have access to bodies of saltwater. The state of California, for example, is largely exposed to the Pacific Ocean, which creates a unique situation in which they may utilize this source of salt water for potable water through desalination. In this case, due to its access to both fiscal support from the federal government, and local infrastructure, the state of California is able to implement reverse osmosis systems (RO) [5]. As of 2012 two desalination plants were established and 17 more are planned to be constructed to supply a total of 7% of urban water needs [5]. Many of these plants can produce up to 16 million gallons per day; however, this comes at a cost of over $165 million to construct one plant and a yearly maintenance cost of over $10 million per plant [2, 5]. While this is an effective solution for MDCs, lesser developed countries (LDCs) do not have efficient fiscal means to produce nor upkeep a plant like this. In addition, the 17+ planned plants in California are tied to a local or state water service, meaning they have the means and infrastructure to distribute the desalinated water, while LDCs do not [2-4].
To address this growing problem, there has been an increased focus on using passive desalination to create an affordable and realistic potable water source for LDCs. One specific technology that has recently garnered interest is MD. This method of desalination is passive, meaning that, in contrast to RO, no mechanical, electrical, nor infrastructural resources need to be used for it to function [1]. While this technology is inefficient compared to RO (in terms of water production, speed, and scalability), its passivity, minuscule size, and portability, makes MD much less of a capital investment and lowers maintenance costs [1, 5].
Here, we designed a passive solar still, that utilized membrane distillation, that was capable of relatively high-water flux, low but stable conductivity, and a high ΔT between the heat source and bottom layer, all of which is necessary for water desalination to occur. Additionally, unlike a traditional MD system, the still needs to wick its water from its source (salt water), which allows for a continuous supply of salt water. In summary there needs to be three stages to this system: a top hydrophilic layer attached to a wick that sits in the source water, a second hydrophobic layer in order to create a vapor flux, capable of deriving water while leaving NaCl behind, and a third hydrophilic layer to condense the water vapor, attached to a wick to transport the distillate into a receptacle for use. In addition to the traditional still design, this still will be modular and flexible in order to ensure portability. Furthermore, to achieve small design the three stages (hydrophilic, hydrophobic, and hydrophilic) will be composed in one singular membrane with three distinct materials (the 3S Membrane), in order to ensure flexibility.
MATERIALS AND METHODS.
Membrane Fabrication.
To fabricate one cohesive membrane, electro spinning was used as the method to fabricate the three distinct stages. First, a “dope solution” (polymer dissolved in a solvent) was created. Once the polymer of choice has been dissolved in a solvent, it is loaded in a syringe and pushed through a positively charged needle aimed at a negatively charged spinning drum. After enough fibers have been spun on the drum, it creates a cohesive membrane.
In this specific case, three dope solutions were spun consecutively in order to fabricate one membrane with three distinct layers: hydrophilic, hydrophobic, and hydrophilic, respectively. Two dope solutions were used in order to create the hydrophilic and hydrophobic layers. For the hydrophilic layers, 12 wt% polyacrylonitrile (PAN) was dissolved in 88 wt% DMF (Dimethylformamide); for the hydrophobic layer, 20 wt% poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) was dissolved in 27 wt% Acetone and 52 wt% dimethylformamide (DMF) [6, 7]. Parameters of the electro spinner for the entire process were as follows: x-axial sliding on, 13-15 kV of voltage, 1.2 mL/hr dope solution flow rate, and a drum rotation speed of ~500nrpm.
Solution Welding.
Once the electro spinning stage of membrane fabrication was complete, the pore sizes of the hydrophilic layers were too large to wick water. Essentially, following the principal of capillary action, if the pore size is too large, the liquid entry pressure (LEP) will not be negative enough for the capillary force to overcome gravity. Therefore, to increase the negativity of the LEP, pore size must be decreased.
Solution welding uses a solvent diluted in alcohol in order to “weld” the fibers of the membrane together, in turn, shrinking the pore size (rmax) and increasing the negative magnitude of the LEP. A ratio of 1:25 μL of DMF to ethanol was used following the electrospinning of the 3S Membrane.
3S Membrane Design.
To ensure that the electro spinning of the 3S Membrane did in fact produce three distinct layers, energy-dispersive x-ray spectroscopy was used to confirm that the nitrogen located in PAN and the Fluoride PVDF-HFP were present in their respective layers.
By indicating that the two hydrophilic layers were separate, MD would be possible, as water vapor may pass through the PVDF-HFP while leaving the NACl behind. Additionally, the presence of PAN on the top and bottom after solution welding, wicking and retention of both 3.5 wt% NaCl water and permeate is possible.
3S Membrane Testing.
To test the 3S membrane, it was first put through a MD cell test cycle. This was used to ensure that the membrane was capable of a water flux and producing permeate. Essentially, this was a fail-safe to determine whether or not the membrane was actually desalinating water. Additionally, to ensure that there was no holes or failure in the membrane, a conductivity meter was placed in the permeate flow side to ensure no NaCl passed through the membrane.
After the MD cell test cycle was completed, the membrane was tested in a laboratory bench top setup to determine whether it was capable of passive desalination. In this test there were no pumps creating a constant supply of water, the only non-passive portion of this test was the heat supply. The same salt wt% from the MD cell test were used, in addition to heat difference. Probes were placed below the heat supply and below layer 3 of the member to determine the difference in temperature, as this is essential to water flux.
RESULTS.
Membrane Distillation Cell.
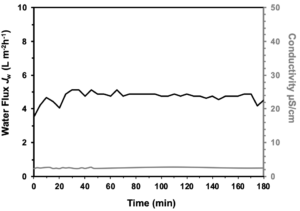
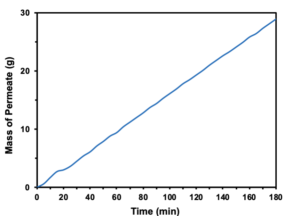
During initial testing of the 3S Membrane in the MD cell testing phase there were constant trends in many of the variables involved. Water flux stayed consistent and at a high rate throughout testing. Conductivity was consistent as well, and remained relatively low throughout testing, indicating the permeated water was fresh (Figure 1). The result was a linear regression of the mass of the permeate, as evident in Figure 2.
Figure 1. Water flux and conductivity as a function of time, of a 3S Membrane during a non-passive membrane distillation test.
Figure 2. Mass of permeate as a function of time, during a non-passive Membrane Distillation test.
Bench Top Laboratory Testing.
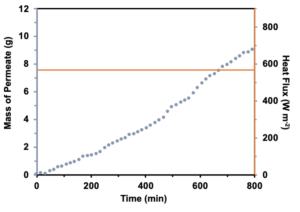
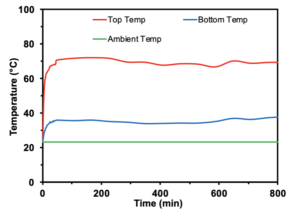
The results of the benchtop style test were largely similar to those in the membrane distillation cell test. Throughout the testing period, there was a linear regression of the mass of the permeate (Figure 3). Also, in order for MD to occur, there must be a difference in temperature, which is indicated in Supplementary Figure 1. The difference in temperature throughout the testing period between the “Top Temp” (temp between the heater and the first layer of the membrane) and “Bottom Temp” (temp under the third layer of the membrane) was ~30 degrees Celsius.
Figure 3. y axis indicates both the mass of permeate and heat flux during a passive bench top test. A startup time of ~30 minutes was demonstrated in the permeate accumulation. After 800 minutes a total mass of permeate of 9 g was achieved.
DISCUSSION.
The consistent water flux is essential as it indicates that the membrane is functional in the MD setup and is capable of desalination with a large enough ΔT in the passive system (Supplementary Figure 1). These results are similar to a previous study as any successful hydrophobic membrane should have a high flux indicating that fresh water is able to evaporate and pass through the membrane [7]. Additionally, the conductivity was low on the permeate side, indicating that the gathering of permeate is due to a high-water flux, and not due to a hole in membrane. The results of this conductivity testing demonstrate that the membrane is actually filtering out the NaCl from the water and producing freshwater.
The mass of the permeated water highlights both the membrane’s efficacy and efficiency. The mass of the permeate shows the extent of the water flux and how the membrane is able to produce distilled water. During this specific MD testing, the membrane produced 30g of freshwater in 180 minutes. While reverse osmosis can generate more permeate, thousands of gallons rather than grams, the membrane developed here produces its permeate passively, facilitating low cost production and maintenance for LDCs [1, 5].
The laboratory bench top testing was significant because it put this membrane and in turn the overall design for a solar desalination device through passive testing. Because the temperature difference was around 30°C, water flux is able to occur. This is significant because water flux is needed in order to pull evaporated water through the hydrophobic membrane [1, 7]. Additionally, the mass of the permeate showed that, while it is less effective than the MD self-test, the passive system is still able to desalinate seawater.
CONCLUSION.
Through the use of membrane distillation, a truly passive alternative for water desalination is possible. While this initial testing of the membrane did not involve solar energy, a future direction would be to add a solar absorber to replace the use of the heater. The results of both the MD cell tests, and bench top lab testing indicate that this membrane design has potential for use by families without access to potable water. This addresses the initial goal of creating availability for families in lesser developed nations, and in turn creates a solution for water desalination in all parts of the world.
ACKNOWLEDGMENTS.
Dr. Nathan Haag acted as a liaison between the School for Science and Math at Vanderbilt, and the Lin research group. Additionally, this project was funded by the Lin research group and the Vanderbilt University Department of Civil and Environmental Engineering. This research co-op was made possible by the School for Science and Math at Vanderbilt and its donners.
SUPPORTING INFORMATION.
Supplementary Figure 1: ΔT in benchtop testing
REFERENCES
- E. Chiavazzo, M. Morciano, F. Viglino, M. Fasano, P. Asinari, Passive solar high-yield seawater desalination by modular and low-cost distillation. Nature Sustainability. 1, 763–772 (2018).
- M. Fasano, Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Commun. 7, 12762 (2016).
- G. Amy, N. Ghaffour, Z. Li, L. Francis, R. V. Linares, T. Missimer, S. Lattemann, Membrane-based seawater desalination: present and future prospects. Desalination. 401, 16–21 (2017).
- J. R Ziolkowska, Desalination leaders in the global market—current trends and future perspectives. Water Sci. Technol. 16, 563–578 (2016).
- Infrastructure Updates – Cal Water. California Water Service. [Online]. Available: https://www.calwater.com/infrastructure-improvements/. [Accessed: 02-Jul-2019]
- H. R. Jung, D. H. Ju, W. J. Lee, X. Zhang, R. Kotek, Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes. Electrochimica Acta. 54, 3630–3637 (2009).
- T. Ogawa, B. Ding, Y. Sone, S. Shiratori, Super-hydrophobic surfaces of layer-by-layer structured film-coated electrospun nanofibrous membranes. Nanotechnology. 18, 165607 (2007).
Posted by John Lee on Wednesday, December 23, 2020 in May 2020.
Tags: Desalination, electrospinning, Membrane Distillation, Solar Desalination, Sustainability