The Association between Social Fear and Intrinsic Amygdala Function Is Modulated by Biological Sex
ABSTRACT
All animals experience various levels of fear. Some experience mild levels and others experience more extreme levels. We proposed that these differences in fear expression result from fundamental distinctions in social processing, suggesting intrinsic anomalies in brain function. The amygdala is responsible for fear mediation in animals and for responses to social stimuli independent of fear stimuli. Therefore, we hypothesized that there is an association between social fear and amygdala activity and that this association is modulated by biological sex. To investigate whether this association exists, we quantified amygdala activity using the amplitude of low-frequency fluctuations (ALFF). We examined this in prepubescent children using magnetic resonance imaging. We found that there was an association between social fear and amygdala activity and that the association was modulated by biological sex. In males, higher social fear was correlated with heightened intrinsic amygdala activity. In females, higher social fear was negatively correlated with heightened intrinsic amygdala activity. The findings from this study have implications for establishing a better understanding of the neurobiological basis of social anxiety disorder; in elucidating these neurobiological markers, researchers can establish better preventative measures and improve current treatment methods for social anxiety disorder.
INTRODUCTION.
All animals experience fear. Fear expression is a survival mechanism. Fear motivates animals to escape predators in threatening situations, and human beings express fear in threatening social situations.
While humans are social beings, individuals vary in social traits. One key trait is social fear. Social fear includes both adaptive and maladaptive fear traits demonstrated in all animals [1]. Every individual ranges along the social fear continuum, from low social fear characterized by outgoing, risk-taking behavior to high social fear characterized by shy, risk-adverse behavior. High social fear can be detrimental to a person’s overall mental health [2-5]. Research on the fear and anxiety neurocircuitry in rodents, non-human primates, and humans [6] points to distinct subcortical regions responsible for social fear expression. Recently, studies in humans (e.g. [7]) have suggested that sex differences affect brain structure and amygdala volume [8]; we believe these sex differences impact brain function, especially in the amygdala.
In the last decade, researchers have been interested in distinguishing key regions of the brain that underlie in social processing. Several core brain regions have been identified, including the amygdala. Associated with undesirable emotional responses, the amygdala is a gray matter mass responsible for the detection and processing of an immediate threat [9]. Numerous studies, such as Etkin & Wager [9], conclude that the amygdala plays a vital role in regulating social anxiety. Avery and Blackford [1] suggested that people with high social fear experience sustained amygdala hyperactivation during a specific task. Previous investigations of the association of amygdala activity and social fear in animals and humans have focused on amygdala activity during tasks or functional connectivity at rest. Investigating distinct traits that may impact amygdala activity at rest helps researchers understand that fundamental basis of the amygdala’s function in the brain, and the association of amygdala activity at rest with social fear has been understudied.
Neuroimaging studies examine social fear and other social traits using magnetic resonance imaging (MRI) methods. MRI technology allows for a non-invasive, in vivo investigation of brain differences. Using functional MRI (fMRI), images are created by measuring the blood-oxygenation level dependent (BOLD) signal, obtained during the task-based responses and the resting-state within the brain. Investigating the resting-state, or intrinsic brain activity, provides a unique perspective into exploring the function of the brain without performing a specific task. The resting-state is believed to elucidate the complexities of baseline brain activity. The amplitude of low-frequency fluctuations (ALFF) is a localized quantification of the low-frequency changes in BOLD signal between 0.01 and 0.08 Hz [10,11] at rest. Low-frequency signals are used in resting state because they measure brain activity specifically and decrease the effect of physiological noise.
This study examined the association between individual differences in social fear and intrinsic amygdala function. Because the amygdala mediates fear response, we believe a relationship between trait social fear and amygdala activity could point to fundamental distinctions in humans ranging along the social fear continuum. We hypothesized that there is an association between high social fear and amygdala activity at rest. We investigated this association in prepubescent children, determining whether the association was modulated by biological sex. We hypothesized that the relationship between social fear and amygdala function was modulated by biological sex.
METHODS.
Participants.
Prepubescent children between the ages eight and ten (N=47) [12] were recruited from the local community for a previous study. Subjects were excluded from this study for numerous reasons: previous or current diagnosis of any psychiatric illness, use of psychoactive medications, MRI contraindications, head trauma, having received treatment for an anxiety disorder, and cognitive deficits. Parents granted informed consent, and children granted assent; the Institutional Review Board (IRB) approved this study [12]. Families were financially compensated.
Child social fear scores were obtained using standard parent-report and self-report questionnaires, the Behavioral Inhibition Questionnaire (BIQ-Child and BIQ-Parent). Subjects ranged along the entire social fear continuum. The parent (typically mother) and child scores were correlated (r=0.60, p< .001), according to the Pearson’s correlation coefficient statistical analysis, so these scores were averaged together as a social fear measure. These averaged scores were used in all analyses.
Data Acquisition and Preprocessing.
Structural and functional MRI images (voxel size= 3x3x3 mm; TR=2 seconds) were collected using a Philips 3-Tesla scanner. Data was collected at rest and during an anticipation task. Only the former was used for this study.
Preprocessing was conducted in the CONN toolbox within SPM 8. Preprocessing steps included realignment and unwarping, slice-time correction, segmentation, normalization, and smoothing (6-mm Gaussian kernel). Further preprocessing included linear detrending.
Data Analysis.
The amplitude of low-frequency fluctuations (ALFF) scores were computed using the CONN toolbox within SPM 8 to acquire a quantification of the baseline amygdala function. Zang et al. [11] and Zou et al. [10] quantified these low-frequency fluctuations by measuring their amplitudes along the power spectrum within a given frequency range. ALFF is a measurement of amygdala activity within a low-frequency range relative to all brain activity within the same frequency range. The frequency range of the power spectrum was restricted to 0.01 to 0.08 Hz – the conventional frequency range for low-frequency fluctuations.
The analyses were restricted to an anatomical mask (Harvard-Oxford atlas; 50th percentile) of the amygdala. We conducted Pearson’s correlation coefficient analyses between left and right amygdala ALFF. Scores were correlated (r = .60, p < .001) between the two hemispheres. Therefore, averaged amygdala scores were used in all analyses. General linear model analyses (e.g. ANOVA) were performed to determine the association between biological sex, social fear, and the interaction between biological sex and social fear on amygdala ALFF scores.
RESULTS.
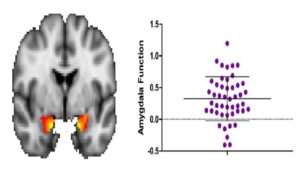
To depict whether intrinsic amygdala activity varied across individuals, we plotted each subject (N=47) on a dot plot according to the subject’s ALFF score. Zero represented the standardized mean ALFF measure relative to the whole brain across all subjects.
Overall intrinsic amygdala function demonstrated substantial variability across the sample (Figure 1). These measurements were calculated by comparing amygdala BOLD signal between 0.01 to 0.08 Hz to the BOLD signal across the brain within the same frequency range. These calculations indicated substantial variability in amygdala ALFF scores, suggesting substantial variability in amygdala function at rest.
Figure 1. Amygdala function at rest measured by ALFF score. Positive and negative ALFF scores illustrate the deviation from the normalized ALFF score, with 0 representing the standardized mean score (see the vertical axis). The dot plot focuses on activation in the amygdala (orange), and each point represents a subject in the study (n=47). Most subjects were between 0 and 0.75.
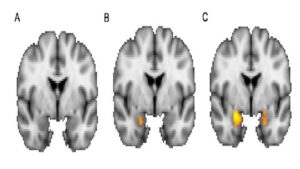
To test our hypothesis, we examined whether biological sex and social fear affected amygdala function at rest (Figure 2A and 2B). We also examined the interaction between biological sex and social fear (Figure 2C). There was less amygdala activation when comparing the sex effect to the ALFF score (Figure 2A). There was more amygdala function in the left amygdala than the right amygdala (Figure 2B). However, the comparison between amygdala function and social fear was stronger than the biological sex effect. There was substantial activation in both amygdala but more in the left than the right across the main effects and the interaction between the main effects. The strongest comparison was demonstrated between amygdala ALFF and the interaction between biological sex and social fear (Figure 2C).
Figure 2. Main effects of (A) biological sex, (B) social fear, and (C) the interaction between biological sex and social fear (F (1,43) =5.18, p =0.03). Activation in the amygdala (orange) was greatest in the interaction between biological sex and social fear.
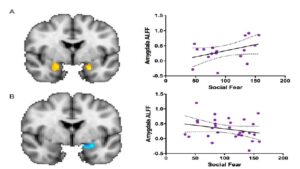
To further investigate the interaction between biological sex and social fear, we divided the dataset into two groups – males and females – and conducted a Pearson’s correlation coefficient statistical analysis.
There was an association between social fear and amygdala function in both males and females (Figure 3). There was a moderately positive correlation (r= 0.41) between social fear and amygdala function (Figure 3A). There was a negative correlation (r= -0.26) between social fear and ALFF scores in females (Figure 3B). This negative effect was noted mostly in the right amygdala.
Figure 3. The association between social fear and amygdala function, or ALFF score, in (A) males (n=17; r= 0.41, p=0.10) and (B) females (n=30; r= -0.26, p=0.17). There was hyperactivation in the amygdala (orange) in males and hypoactivation (blue) in females when comparing the interaction between biological sex and social fear to amygdala ALFF score. Each point represents a subject in the study (n=47). The area between the two curves indicates the confidence interval.
DISCUSSION.
Social fear plays a vital role in how animals respond in social situations. Animals with high social fear demonstrate extreme avoidance behavior, failing to approach novel situations [5]. High social fear is a high-risk factor for some psychiatric illnesses (e.g. social anxiety disorder) [1,2]. Social anxiety disorder (SAD), a prevalent psychiatric illness, develops in early childhood around the age of thirteen and interferes with social interactions and activities [4]. Studying the risk factors for social anxiety disorder and other psychiatric illnesses enables researchers to develop a better understanding of the role of the fear and anxiety neurocircuitry in humans [6].
In our study of intrinsic amygdala activity, we found that the association between social fear and amygdala ALFF scores was modulated by biological sex. Studies have suggested that there are biological characteristics that affect various features in the human brain [7]. Furthermore, previous studies have suggested that there are sex differences in brain structure and function [7]. A study by Cosgrove et al. [8] determined that there are sex-specific regional volume differences (e.g. larger amygdala in males and a larger hippocampus in females) as well as functional differences in humans. Another study found a mediating effect of testosterone on amygdala function in adolescents, thus suggesting sex differences in brain function [13].
In children, our hypothesis that higher social fear would be associated with higher ALFF responses in the absence of external stimuli was supported in males. Young males who express high social fear have not become less socially fearful over time, despite cultural pressures discouraging social fearfulness [14]. Shy males are encouraged to become more outgoing whereas shyness in females is more acceptable [15-20]. This indicates that the social fear we observe in females may be a combination of biology and environment (cultural acceptance of shyness) while the social fear observed in males may more closely reflect biology.
CONCLUSION.
To conclude, we found that the association between social fear and intrinsic amygdala activity is modulated by biological sex. Utilizing MRI data, we quantified amygdala intrinsic activity in children then compared amygdala activity to measures of social. Across the dataset, there was substantial variability in the degree of intrinsic amygdala activity. After testing whether amygdala activity was associated social fear and biological sex, we concluded that there is an association between amygdala activity and social fear, which is modulated by biological sex.
Psychiatric neuroimaging researchers theorize that there are underlying neurobiological mechanisms in psychiatric illnesses. The fear and anxiety neurocircuitry is implicated in many psychiatric illnesses. Previous studies have elucidated the fear and anxiety neurocircuitry in rodents [21,22], non-human primates [23,24], and humans [6]. Several researchers [23,24] describe a trait-like phenotype in non-human primates, called anxious temperament, that represents fearful or avoidant responses to novel social stimuli. To provide for a clear translation from the animal literature on the fear neurocircuitry and anxious temperament [25], researchers study the social fear trait in humans [1,26]. These studies also implicate the amygdala as the central hub of the fear neurocircuitry [6,23].
In the future, because there are many other subcortical regions implicated in social processing (e.g. insula and the bed nucleus of the stria terminalis) [27], we could examine ALFF in those brain regions and compare intrinsic brain function of these individual subcortical regions to that of the amygdala. Additionally, we hope to investigate whether the association between social fear and intrinsic amygdala activity is modulated by developmental stage. The experiences of young children do not compare to the variety of social experiences (e.g. dating and marriage) that most adults encounter that impact social processing mechanisms. We hypothesize that developmental stage would moderate the relationship between social fear and intrinsic amygdala function.
To date, many researchers have studied age differences in brain structure and function; evidence supports that adolescents have greater gray matter volume than prepubescent children. Additionally, the onset of puberty influences amygdala volume differences between sexes [26] and affects regional function [8,27- 29]. Therefore, developmental stage, in addition to biological sex, may play a key role in both structural and functional brain differences. It is important to examine both biological sex and age differences to develop a better understanding of the neurobiological basis of various psychiatric illnesses [7,28] and the fundamental distinctions in social processing.
Discovering the anomalies in intrinsic brain function may aid in the risk reduction of mental illnesses, thus reducing the risk of future psychopathology [6,26]. The fundamental differences demonstrated in our study indicate possible neurobiological markers for social anxiety disorders and other psychiatric disorders in humans [1]. In determining these neurobiological markers, researchers can focus on establishing better preventative measures against and improving current treatment methods for social anxiety disorder [6]. Individuals who suffer from social anxiety disorder usually experience both fear of judgment and negative self-perception. This neuroticism establishes feelings of fear, depression, anxiety, envy, and frustration [31]. These feelings inhibit the ability to participate in society as intellectual, social beings.
ACKNOWLEDGMENTS.
Thank you to Dr. Christopher Muller and the School for Science and Math at Vanderbilt as well as the members of the Blackford Lab for their continuous support.
REFERENCES.
- S.N. Avery, J. U. Blackford, Slow to warm up: the role of habituation in social fear. Social Cognitive and Affective Neuroscience. 11, 1832-40 (2016).
- V. Miskovic, L. A. Schmidt, Social fearfulness in the human brain. Neuroscience and Behavioral Reviews. 36, 459-78 (2012).
- J. A. Clauss, J. U. Blackford, Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. Journal of the American Academy of Child & Adolescent Psychiatry. 51, 1066-75 (2012).
- J. U. Blackford et al., Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biological Psychology. 99, 15-25 (2014).
- J. A. Clauss, S. N. Avery, J. U. Blackford, The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Progress in Neurobiology. 127-128, 23-45 (2015).
- J. A. Clauss et al., Neurocircuitry underlying risk and resilience to social anxiety disorder. Depression and Anxiety. 31, 822-33 (2014).
- L. Cahill, Why sex matters for neuroscience. Nature Reviews Neuroscience. 7, 477-84 (2006).
- K. P. Cosgrove, C. M. Mazure, J. K. Staley, Evolving Knowledge of Sex Differences in Brain Structure, Function, and Chemistry. Biological Psychiatry. 62, 847-55 (2007).
- A. Etkin, T. D. Wager, Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American Journal of Psychiatry. 164, 1476-88 (2007).
- Y. Zang et al., Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 29, 83-91 (2007).
- Q. Zou et al., An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. Journal of Neuroscience Methods. 172, 137-41 (2008).
- J. A. Clauss et al., Altered Prefrontal Cortex Function Marks Heightened Anxiety Risk in Children. Journal of American Academy of Child & Adolescent Psychiatry. 55, 809-16 (2016).
- M. Ernst et al., Amygdala Function In Adolescents With Congenital Adrenal Hyperplasia: A Model For The Study of Early Steroid Abnormalities. Neuropsychologia. 45, 2104-13 (2007).
- J. M. O’Neil et al., Gender-role conflict scale: College men’s fear of femininity. Sex Roles. 14, 335-50 (1986).
- X. Yang et al., Intrinsic Brain Activity Responsible for Sex Differences in Shyness and Social Anxiety. Frontiers in Behavioral Neuroscience. 11, 43 (2017).
- J. G. Allen, M. Haccoun, Sex Differences in Emotionality: A Multidimensional Approach. SAGE Journals. 29, 711-22 (1976).
- A. Fischer, Gender and Emotion: Social Psychological Perspectives (Cambridge Univ. Press, Cambridge, UK, 2000), pp. 24-47.
- F. C. Gerull, R. M. Rapee, Mother knows best: effects of maternal modelling on the acquisition of fear and avoidance behaviour in toddlers. Behavioral Research and Therapy. 40, 279-87 (2002).
- M. S. Horner, Toward An Understanding of Achievement-Related Conflicts in Women. Journal of Social Issues. 28, 157-75 (1972).
- J. M. O’Neil, Patterns of Gender Role Conflict and Strain: Sexism and Fear of Femininity in Men’s Lives. Journal of Counseling & Development. 60, 203-10 (1981).
- C. Qi et al., Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes, Brain and Behavior. 9, 974-84 (2010).
- A. S. Fox et al., Extending the amygdala in theories of threat processing. Trends in Neuroscience. 38, 319-29 (2015).
- A S. Fox et al., Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS one. 3, e2570 (2008).
- J. A. Oler et al., Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 466, 864-68 (2010).
- A. S. Fox, N. H. Kalin, A Translational Neuroscience Approach to Understanding the Development of Social Anxiety Disorder and Its Pathophysiology. The American Journal of Psychiatry. 171, 1162-73 (2014).
- J. U. Blackford, D. S. Pine, Neural Substrates of Childhood Anxiety Disorders: A Review of Neuroimaging Findings. Child and Adolescent Psychiatric Clinics of North America. 21, 501-25 (2012).
- S. N. Avery, J. A. Clauss, J. U. Blackford, The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology. 41, 126-41 (2016).
- S. J. Blakemore, S. Burnett, R. E. Dahl, The role of puberty in the developing adolescent brain. Human Brain Mapping. 31, 926-33 (2010).
- E. E. Nelson, Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. Journal of Child Psychology and Psychiatry. 44, 1015-24 (2003).
- R. E. Dahl, M. R. Gunnar, Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 21, 1-6 (2009).
- T. L. Rodebaugh et al., The structure of vulnerabilities for social anxiety disorder. Psychiatry Research. 250, 297-301 (2017).
Posted by John Lee on Tuesday, December 22, 2020 in May 2018.
Tags: ALFF, amygdala, social anxiety disorder, Social fear