Review: Outstanding hazards of radionuclides from the West Lake Landfill
ABSTRACT
The West Lake Landfill, located just outside St. Louis, Missouri, began as a quarry but became an illegal dumping site for hazardous chemicals and radioactive substances. Home to radiation, the West Lake Landfill serves as a final resting point for radionuclides that are raising health inquiries for those living and working in the area. Despite many steps having been taken to remedy this issue, the primary concern of off-site contamination remains. However, there are various steps that can be taken to protect oneself from exposure to radium and radon, the major radioactive chemicals of concern. There is a lack of synthesis of the history and major concerns of the landfill. This research is aimed to provide an encompassing view of the hazards of radiation remaining in St. Louis County, MO.
INTRODUCTION AND HISTORY.
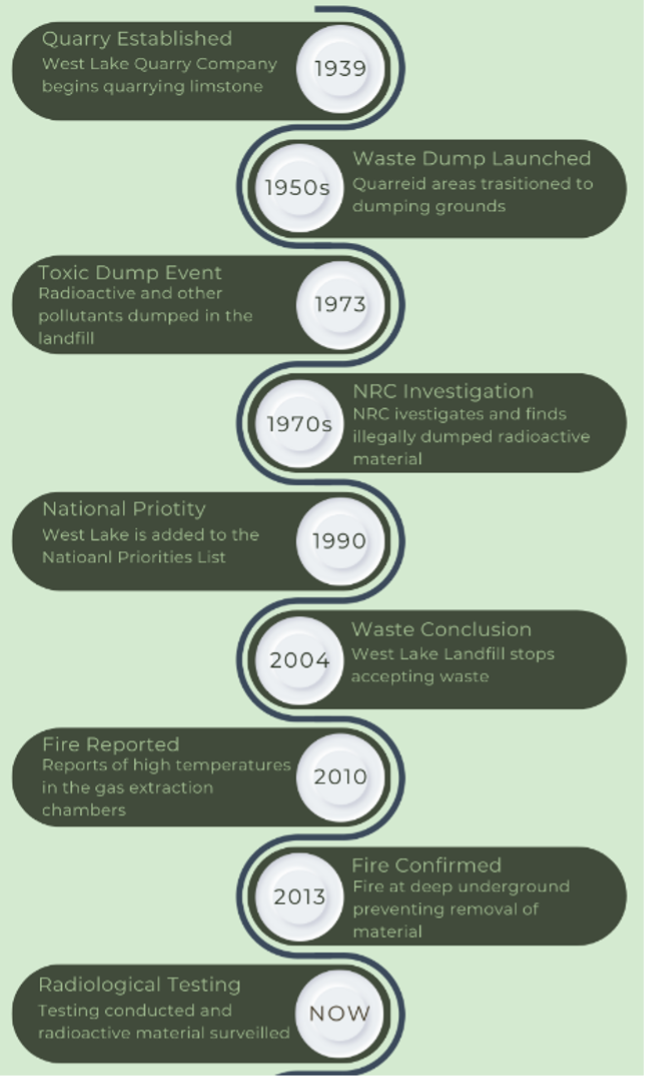
The Westlake Quarry Company quarried limestone from the Bridgeton, Missouri area between 1939 and 1987 (Figure 1) [1]. These quarried areas eventually became home to many tons of radioactive waste produced from the Manhattan Project. Radioactive substances leaked into Coldwater Creek, which flows into the Missouri River and contaminated both the groundwater and soil in the surrounding area, which is home to a multitude of residential and commercial communities [2].
Figure 1. West Lake Landfill Timeline. The landfill began as a limestone quarry and overtime converted to a radioactive dumping ground. Today the contamination is location under a massive tarp and a multitude of gas wells for resident safety. The figure was created from data found from the Missouri Department of Natural Resources [7].
1930-1940s. The Manhattan Project began in 1942. The project was codenamed for the US-led effort to create a functional atomic weapon during World War II [1]. Nuclear plants were built across the country to rapidly build atomic weapons, and St. Louis was the city chosen for uranium refining. Uranium is isolated from rock deep beneath the earth’s surface. The isolated uranium forms the atomic weaponry used in World War II and nuclear power plants. Radioactive waste is a byproduct of nuclear reactors, fuel processing plants, hospitals, and research facilities. The Mallinckrodt Chemical Company began processing uranium and other nuclear materials in St. Louis for the Manhattan Project.
1950-1990s. After the end of the Manhattan Project in 1947, more than 168 tons of uranium waste products remained [1]. 92 percent of these waste products were shipped to Cañon, Colorado. The remaining 8 percent remained at the site [1]. In the 1950s, portions of quarried areas from Westlake Quarry Company and surrounding areas were used to dispose of solid municipal waste and industrial waste along with construction and demolition debris. Radioactive waste produced from the Manhattan Project was moved to nearby at the Latty Avenue site which is north of the St. Louis-Lambert International Airport [5]. The waste storage at both the Westlake and Latty Avenue sites, causing leakage into Coldwater Creek, which lies nearby many residential areas as it seeks the Missouri River. Following a Nuclear Regulatory Commission investigation of illegally stored radioactive material in 1973, 8,700 tons of radioactive barium sulfate were discovered to have been illegally dumped in the West Lake Landfill [7]. These substances were mixed with 12-18 inches of topsoil [1]. When mixed with the topsoil, the materials, although diluted, became more difficult to locate as the material dispersed itself within the site.
Limestone quarrying was completed in 1987 (Figure 1) and in the early 1990s the West Lake Landfill was added to the Environmental Protection Agency (EPA) National Priorities List. The West Lake Landfill was declared a Superfund Site, which is a polluted location in the US requiring a long-term response to clean up hazardous material contaminations (Figure 1) [6]. Currently there are 1,344 Superfund Sites in the United States, most of which are in areas of high industrial activity.
2000-Present. The West Lake Landfill Superfund Site stopped accepting all waste on December 31st, 2004 (Figure 1) [7]. Based on the hazard presented, the major focus is now to contain or remove the hazardous materials present. Installing a tarp on top of the dumped material was the first step to protecting the local area. The tarp blocks radionuclides and other noxious chemicals from diffusing into the air. Gas extraction wells were also placed on top of the landfill to reduce the toxic fumes produced from decaying material under the tarp [7]. The tarp functions as a protection for nearby communities.
In December of 2010, landfill workers reported elevated temperatures from some of the gas extraction wells. In March 2013, a fire was confirmed. The fire was over 150 feet deep and covered an area of nearly 15 football fields. Officials decided to let the fire smolder, as excavating it could cause an increase in toxic fumes in the area and bring the fire to the surface [7].
The original plans from 2018 were to excavate the radioactive material by 2022 [7]. Currently in 2022 the Superfund Site is still in the planning phase. The stall in these clean-up efforts is largely due to the global COVID-19 pandemic. Officials continue to test the soil of the landfill for radioactive contamination. As of November 9th, 1,000 locations have been sampled on the landfill’s property. Of these 1000 samples 443 are radiologically contaminated. Thirty-nine of the sampled locations are pending results [8].
The clean-up efforts are split into three operable units for timeline clarity and reasonable goals. Operable Unit One focuses on the cleanup of radiological material. The contamination of former waste disposal areas is addressed in Operable Unit Two. Operable Unit Three is devoted to statewide groundwater contamination [7]. Progress has been made to clean up the landfill for both operable units one and two. The landfill is sectioned into different operable units in which to plan landfill cleanup. The current plans for OU1 include excavating radiologically impacted material greater than a level of 52.9 pCi/g 12 feet below the surface topographic depth and excavation of radiologically impacted soil from several areas, along with fast removal of contaminated material [7]. These materials will then be relocated to an off-site permitted disposal facility. Current plans of OU2 include long-term surveillance, institutional controls, and land monitoring [7]. For the most recent updates on the West Lake Landfill clean up visit the EPA’s West Lake Landfill Dashboard [8].
EVERLASTING CONTAMINANTS.
Many chemicals of concern were disposed of in the landfill. These include inorganic chemicals, pesticides, polychlorinated biphenyls, and semi-volatile or volatile compounds. Inorganic compounds of concern are antimony, arsenic, barium, beryllium, chromium, cobalt, lead, mercury, nickel, thallium, uranium metal, vanadium, and zirconium [7]. Pesticides and polychlorinated biphenyls of concern are aldrin or dieldrin, aroclor 1242, aroclor 1248, and aroclor 1252. Semi-volatile to volatile organic compounds of concern are benzene, chlorobenzene, 1-dichloroethane, 4-dichlorobenzene, ethylbenzene, naphthalene, and pentachlorophenol [7]. Many of the listed chemicals are either carcinogenic when inhaled or toxic when inhaled.
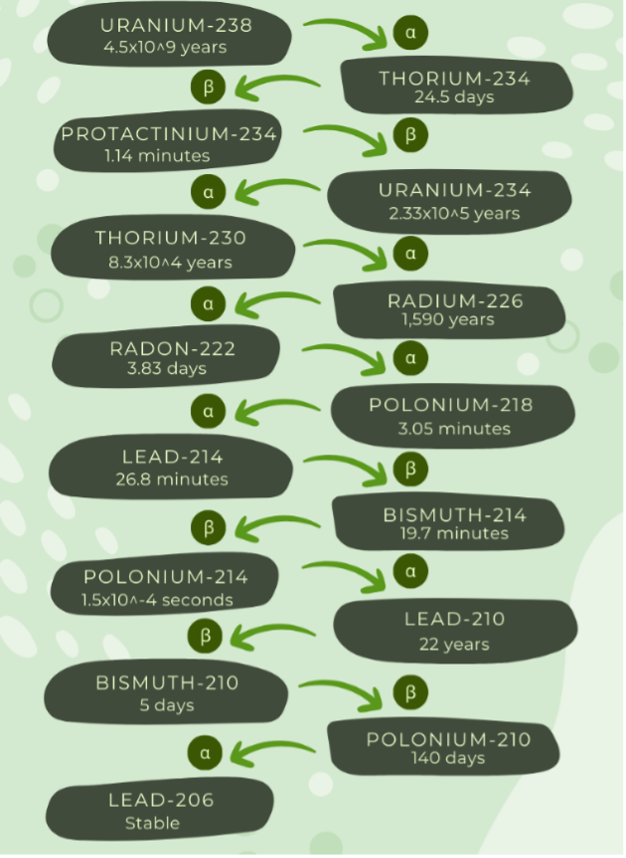
238-Uranium (238U) is a major radioactive chemical of concern with a half-life of 4.5 billion years. As it undergoes alpha decay, 238U decays to 230-thorium (230Th), which has a half-life of approximately 75,000 years. As 230Th undergoes alpha decay, it decays to 226-radium (226Ra), which has a half-life of 1,600 years [9]. 226Ra emits 222-radon as it decays, which can build up in poorly ventilated buildings or structures in the surrounding areas [9]. Therefore, a major concern for those located around the site is groundwater contamination of other chemicals at the landfill and radon buildup in their houses.
Radium. Radium is the 88th element on the periodic table of elements. Radium is known as the only radioactive alkaline earth metal. All isotopes of radium are radioactive; however, 226Ra has the longest half-life [9]. In Figure 2, as radium decays, it emits ionizing radiation, and its “daughter” is radon gas [9][10]. Ionizing radiation removes electrons from nearby matter. Ionizing radiation includes gamma rays and x-rays. In nature, radium is found in uranium and thorium ores and is produced naturally within the earth in minute amounts.
Figure 2. Uranium decays to lead through a multistep process. Each step within the chain of decay occurs spontaneously over varying time spans. Beta decay has more potential harmful effects than alpha decay. The chain ends with lead, which will not decay further. Figure data accessed from the Canadian Nuclear Safety Commission [10].
Radium was isolated in its better-known metallic state by Marie Curie and Andre-Louis Debierne through the electrolysis of radium chloride in 1911 [11]. Marie Curie dedicated her life to studying radium and is thought to have died due to its radiation [11]. When attached to Pierre Curie’s arm in a vial, radium was blamed to have caused a skin lesion after ten hours [12]. In remembrance of their work, the international standard radiological unit of measure became known as “curies” [13].
Many companies employed workers known as radium girls during World War I. During World War I many companies employed girls to paint everyday objects with “anti-dark”. These girls became known as the radium girls for their ingestion of radium. This illumination would not go out unlike typical luminous creations of the present day. The girls were instructed to give the brushes, used to paint the watches, a fine point by licking them [14]. Each girl would ingest around 100-1000 microcuries of radium per year [14]. The exposure to radium gave them sores and caused anemia and bone cancer [14].
Radium will enter the body when inhaled or swallowed. 80 percent of ingested radium leaves the body in feces while the remaining 20 percent enters the bloodstream [15]. The body treats radium similarly to calcium, depositing it into the bones. The radioactive nature of radium causes death or mutation of nearby cells. When inhaled, radium particulates will remain in the lungs for months and gradually enter the bloodstream. Over time, prolonged exposure increases risks of cancer of both the bone and lung [14]. High dose exposure can cause anemia, cataracts, broken teeth, and reduced bone growth [15]. High levels of radium are found in waste from former radium processing and manufacturing facilities [9].
Radon. As shown in Figure 2, radon is a direct breakdown product of radium decay. Radon (Rn) is the 86th element on the periodic table and is one of only two radioactive noble gasses. It is a colorless, tasteless, odorless gas of which all isotopes are radioactive [15]. 22Rn has a half-life of 3.8 days, then decays into its final form of 206-Lead (210Pb) [11]. Due to radon’s low half-life, there have been few controlled experiments to study its health effects. It is known to be the second highest contributor to lung cancer, the largest contributor to individual background radiation, and is currently the leading environmental cause of cancer mortality in the United States [15].
Radon was first discovered by Fredrich Dorn in 1900 [16]. It was originally named nitens for the Latin word for shining. It was renamed to radon in 1923. Radon was believed to be the “poor man’s radium”. After World War II many uranium miners began developing lung cancer due to the lack of occupational limits for radon concentrations within the mines [16]. Until the mid-1980s radon was not thought to be a potential public health risk in the home [16]. In response, the EPA began a proficiency program to make protective publications nationally available. The program was discontinued in 1998, however, current guidelines for radon levels are labeled by the National Radon Safety Board or the National Radon Proficiency Program of the American Association of Radiation Safety Technicians [16].
Radon is commonly found in uncovered dumps, airtight buildings, and tailing ponds, man-made reservoirs under which toxic waste is stored [16]. Even though people are commonly exposed to radon, it can easily be prevented. Refraining from smoking and increasing airflow in the house will prevent stagnant vapors [18]. Frequent cleaning and dusting can also help prevent buildup of contaminated soil particulates. Sealing cracked floors and walls with plaster or caulk will prevent radon gas from seeping into a basement [17]. Utilizing radon-resistant construction techniques can lower radon exposure and keeping radon test kits in homes and businesses will alert to its presence.
DISCUSSION.
Though the West Lake Landfill began as a limestone quarry, through an illegal dumping it became the irradiated site it is today. The radiated material will continue to be around for many centuries to come.
Recent studies have used scanning electron microscopy (SEM) and energy dispersal x-ray spectroscopy (EDS) to excite the excessive electrons from radioactive elements by beaming more electrons into them [18,19]. This causes inner shell electrons to rise and fall back into ground state releasing photons making them more visible.
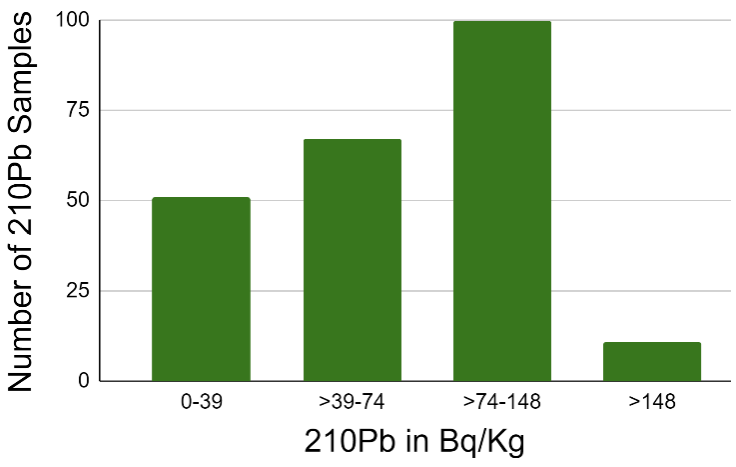
Researchers have traced the number of radionuclides as they spread away from the West Lake Landfill and Coldwater Creek. In their study Kaltofen, Alvarez, and Hixson sought to define the rate of unsupported 210Pb from buried uranium and radium [19]. The unsupported 210Pb is directly caused by the decay of radium to radon and finally to lead. The group compared their results to 1978 reports of radiation from the landfill. They noted that the Latty Avenue 230Th were similar in activity to 1987 230Th activity averages from the Latty Avenue waste pile [19]. Therefore, the long-term hazard of the radioactive alpha decay at the site was not decreasing as expected based on the half-life of 230Th. The group collected dust particulates from the home and analyzed each sample using SEM/EDS. Samples indicated contamination of material like the uranium processing wastes from the Mallinckrodt Chemical Company [18]. Table 1 and Figure 3 shows the percent of unsupported 210Pb in comparison to the activity levels of 210Pb and 226Ra. Unsupported 210Pb is lead that lacks a clear source of origination. Table 1 indicates the percent of 210Pb originating from an outside source in comparison to the total 210Pb found. As the uranium chain decays, 210Pb serves as a marker for excess 222Rn. As a daughter of 226Ra, 222Rn displays the levels of 226Ra at the collection site. The 210Pb activity level in figure two shows the number of samples that exceeded acceptable levels of lead. Table 1 and Figure 3 presents an unnatural level of lead collection in the house, likely originating from the 226Ra activity. The data shows that the radionuclides moved from their original sites. These chemicals are now going through alpha decay in populated areas.
| Table 1. Samples taken from numerous homes near Coldwater Creek contain unnaturally high levels of 210Pb. 210Pb and 226Ra were measured using SEM/EDS technology. 210Pb and 226Ra activity shown as radioactive decay per kilogram (Bq/Kg) highlights the percent unsupported 210Pb, meaning 210Pb which is above background level. Seven of the nine homes indicate 226Ra decay given by the percent of unsupported 210Pb. House E is calculated from the 226Ra detection limit. An increased percent of unsupported 210Pb suggests 226Ra contamination originated from an outside source. Data from table reproduced from reference 18. | |||
| Sample ID | 210Pb (Bq/ Kg) | 226Ra (Bq/Kg) | % of unsupported 210Pb |
| House A | 3790 | 286 | 93 |
| House B | 445 | 23.2 | 95 |
| House C | 360 | 16.8 | 95 |
| House D | 347 | 17.3 | 95 |
| House E | 271 | <5 | >96 |
| House F | 263 | 25.3 | 90 |
| House G | 225 | 11.2 | 95 |
| House H | 95 | 43.4 | 54 |
| House I | 74 | 38 | 49 |
Figure 3. Soil Samples from homes near Coldwater Creek tested for 210Pb. Samples were analyzed using SEM/EDS technology. The graph represents the number of samples within the range of 0->148 Bq/kg. 77.7% exceeded background levels of 0-39 Bq/Kg, exceeding background. Graph reproduced from data from reference 18.
In a secondary study Kaltofen, Alvarez, and Hixson sought to trace specific radionuclides as they spread around St. Louis County. They used SEM/EDS technology to identify radioactive particulate matter that diffused through the environment. Uranium, thorium, and radium were studied. They show that the 226Ra found in soil samples is not sufficient to form the 210Pb also located within the samples. This misalignment shows the radon found in the samples must be from a parent outside of the source. “More than 90% of the 210Pb in seven of the 13 house dust sampled near Coldwater Creek implies that there is an external source of 222Rn that is impacting the indoor environments at these homes’” [19]. Around Coldwater Creek there are high amounts of uranium and thorium. Settlements at known sites potentially have lesser levels of uranium and thorium than houses nearby the creek [19]. This data alludes to a strong link between the gross radiochemical analysis and historic data from Mallinckrodt uranium processing wastes [19].
Recently, in October of 2022, Kaltofen and Brian Moore tested for radioactive contamination at Jana Elementary School in Hazelwood, MO. The United States Army Corp of Engineers (USACE) reported radioactive activity exceeding the natural background, but below remedial standards as set by Formerly Utilized Sites Remedial Action Program [2]. The reports propelled the hiring of Kaltofen’s team as a third party. The USACE tested only outdoor spaces failing to assess the risks to students and staff. The team took 32 total soil samples, including dust and plant materials [20]. Of the soil samples, locations consisted of the ballfields, Coldwater Creek, and the creek sediments. Sample locations included locations such as the school kitchen, play apparatus, and classrooms [20]. Analysis of the 32 samples was done by Eberline Analytical and Microvision Laboratories. The analysis of samples was taken for radioactive activity, not to be mistaken for dose. It was found that “…the amount and type of radiation found at Jana is well above the standards the EPA has set for the protection of human health” [2]. Kaltofen has taken 540 samples of 210Pb throughout the St. Louis area. Of those samples, only four contain an activity greater than that found at Jana Elementary [2]. He continues to assert that the level of 210Pb found at Jana is related to Coldwater Creek floodings. The floodwaters draw throinated particles tied to the Manhattan project into homes along the banks of the creek [20]. “…inhaling or [ingesting] these particles could subject one to long-term internal exposure to alpha-radiation emitting microscopic particles” [20]. As humans are exposed to radioactivity through the basement, increased contact to this radiation is of concern. Kaltofen concludes his investigation of Jana calling for complete remediation of both Coldwater Creek and Jana Elementary [20]. Temporary decontamination is easily nullified due to the creek flooding.
Exposure Prevention. Everyone is exposed to extremely low levels of radium because it is naturally present in soil, water, rocks, coal, plants, and food [9]. Local governments and environmental agencies are responsible for the protection of citizen and environmental health, especially in radiologically contaminated areas. However, in areas such as the Hazelwood Interim Storage Site there is a lack of physical barriers or signage indicating radiological and hazardous material. Signage is critical to the protection of locals, who may be unaware of the location’s contents or hazards.
At the individual level, testing the home, particularly the basement, for radon is the best way to prevent long-term radium exposure. The half-life of radon, and being the immediate daughter to radium, allows it to be noticed faster than radium. Since radium and radon both diffuse out of soil, the basement will be the first place with radium exposure [17]. The home can also be tested for radioactivity. The tests will not tell what the harmful effects of the exposure might be.
CONCLUSION.
The Westlake Landfill, in Bridgeton, MO, is a site of radioactive substances and hazardous chemicals form the Manhattan Era uranium processing. The radioactive material at the landfill will continue to decay. Therefore, timeline analysis conjoined with research analysis is necessary. The largest threat, radon, will continue to decay and contaminate due to its low half-life and tendency to collect as dust in local residencies. Human health is of major concern when living near these sites, however the risk remains largely unknown. The recent events from Jana Elementary highlight the hidden, yet continuous hazards of radioactive contamination. An effort to decontaminate the sites permanently is the only way to prevent continued contamination for the coming centuries.
ACKNOWLEDGEMENTS.
Figures 1 and 2 were created using Canva design templates. A special thank you to Jenna Zuromski, who mentored me throughout the process of researching and writing.
REFERENCES
- 1. “One Park, Three Sites, Countless Stories.” National Park Service, Available at: https://www.nps.gov/mapr/index.htm, (Accessed: Nov. 18, 2022).
- M. Kaltofen, “Supplemental Expert Report by Dr. Marco Kaltofen Comparing Jana School Radioisotope Results – USACE, Boston Chemical Data, and SCI” (Boston Chemical Data Corp., 2022).
- N. R. Commission, “Radioactive Material in the West Lake Landfill” (Tech. Rep. NUREG-1308, Nuclear Reg. Comm., 1988).
- “Storage and Disposal of Radioactive Waste.” World Nuclear Association, 2022. Available at: https://world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-waste/storage-and-disposal-of-radioactive-waste.aspx, (Accessed: Nov. 18, 2022).
- 5. “Brief History of Radioactivity in metro-St. Louis.” Missouri Coalition for the Env., 2018. Available at: https://moenvironment.org/blog/brief-history-of-radioactivity-in-st-louis/, (Accessed: Nov. 18, 2022).
- 6. “What is Superfund?.” Environmental Protection Agency, 2021. Available at: https://www.epa.gov/superfund/what-superfund, (Accessed: Nov. 18, 2022).
- “West Lake Landfill.” Missouri Department of Natural Resources. Available at: https://dnr.mo.gov/waste-recycling/sites-regulated-facilities/federal/west-lake-landfill, (Accessed: Nov. 18, 2022).
- “West Lake Landfill Dashboard.” Environmental Protection Agency, 2021. Available at: https://www.epa.gov/mo/west-lake-landfill-dashboard, (Accessed: Nov. 18, 2022).
- “Radionuclide Basics: Radium.” Environmental Protection Agency, 2022. Available at: https://www.epa.gov/radiation/radionuclide-basics-radium, (Accessed: Nov. 18, 2022).
- “Radon in Canada’s Uranium Industry.” (Canadian Nuclear Safety Commission, 2012).
- “Marie Curie.” The Nobel Prize. Available at: https://www.nobelprize. org/womenwhochangedscience/stories/marie-curie, (Accessed: Nov. 18, 2022).
- “N. Fröman, Marie and Pierre Curie and the discovery of polonium and radium.” The Nobel Prize, 1996. Available at: https://www.nobelprize.org/prizes/themes/marie-and-pierre-curie-and-the-discovery-of-polonium-and-radium/, (Accessed: Nov. 18, 2022).
- “How the Curie Came to Be.” Oak Ridge Associated Universities, 1996. Available at: https://orau.org/health-physics-museum/articles/how-the-curie-came-to-be.html, (Accessed: Nov. 18, 2022).
- “Radium Girls.” Preservation Snapshot.
- “Radionuclides Basics: Radium.” Environmental Protection Agency, 2022. Available at: https://www.epa.gov/radiation/radionuclide-basics-radium, (Accessed: Nov. 18, 2022).
- Radon History. Virginia Department of Health, 2022. Available at: https://web.archive.org/web/20150211154556/http://www.epa.gov/radiation/radionuclides/radium.html#affecthealth, (Accessed: Nov. 18, 2022).
- “Radon and Your Health.” Centers for Disease Control and Prevention, 2022. Available at: https://www.cdc.gov/nceh/features/protect-home-radon/index.html, (Accessed: Nov. 18, 2022).
- M. Kaltofen, R. Alvarez, L. W. Hixson, Forensic microanalysis of Manhattan Project legacy radioactive wastes in St. Louis MO. Applied Radiation and Isotopes 136, 143-149 (2018).
- M. O Kaltofen, R. Alvarez, L. Hixson, Tracking legacy radionuclides in St. Louis, Missouri, via unsupported 210Pb. J Environ Radioact. 153, 104-111 (2016).
- M. Kaltofen “Radioactive contamination at the Jana Elementary School, Hazelwood, MO: Review of community and USACE radioisotope data” (Boston Chemical Data Corp., 2022).
Posted by John Lee on Tuesday, May 30, 2023 in May 2023.
Tags: Radioactive waste, Radionuclides, West Lake Landfill