Reprogramming adult mammalian Müller glia via L-Myc ex vivo
ABSTRACT
This paper analyzed the use of the transcription factor L-Myc as a method of reprogramming Müller glial cells for therapeutic use following retinal injury. The main goal of this study was to determine if L-Myc overexpression would cause reprogramming and/or proliferation of Müller cells, utilizing Otx2 and BrdU as markers. Multiple time points were also studied to determine the extent and duration of proliferation of reprogrammed cells. Following a novel electroporation technique, immunostaining, fluorescent imaging, and Imaris quantification, it was found that there was a significant increase in Otx2 expression and BrdU incorporation. Furthermore, it was determined that later timepoints (6-7 days post-transfection) had far greater occurrence of proliferation and reprogramming than earlier timepoints (2-4 days post-transfection). These findings suggest that L-Myc may be an ideal candidate for further testing in retinal regeneration studies targeting other retinal neurons.
INTRODUCTION.
Degenerative eye diseases affect over 200 million people annually [1]. Many of these affect the retina, including Age-Related Macular Degeneration (AMD), Diabetic Retinopathy (DR), Retinitis Pigmentosa (RP), and more, typically leading to irreversible vision loss [2]. These are caused mainly by dysfunction in the layers of cells in the retina (retinal pigment epithelium [RPE] cells, Müller glial [MG] cells, ganglion cells, etc.), which either break down or are unable to proliferate, leading to a reduction in vision quality and potentially blindness [3]. Retinal degeneration can be caused by a variety of means, which could be genetic, environmental, age-related, or injury-caused (usually blunt force trauma). These processes cause the retina to be unable to function by inhibiting a specific cell layer, either preventing proliferation or actively causing breakdown, resulting in progressive vision loss [4]. Many of these retinal diseases mirror injury models, meaning that injury models can be utilized to develop cures for retinal diseases.
Müller glia, the major glial cell type of the retina, are responsible for providing structure to the retina and aiding in metabolic processes. Retinal degeneration affecting Müller glial cells causes reactivity and formation of glial scars in order to prevent further damage. However, MG are most significant because they have many progenitor-like characteristics. In vitro, they are capable of being converted to induced pluripotent stem cells (iPSCs), which can be used to change ultimate cell fate [3]. In zebrafish, MG can be used to differentiate into various progenitor cells, which can further differentiate into various retinal cell layers, even including photoreceptors [2]. However, this regenerative capability is notably not present in humans and other mammals such as mice. Zebrafish Müller glia are extensively studied to understand how the signaling pathways present can be used as a model to be replicated in regeneration in mammalian Müller glia [5].
Differentiated cells can be transformed into stem-like cells using Yamanaka factors. These four transcription factors (Oct4, Klf4, Sox-2, and C-Myc) are potent cell reprogrammers (present in embryonic stem cells) that are capable of returning specialized adult somatic cells to a pluripotent stem cell state in a process known as dedifferentiation. These factors serve to mitigate the ethical concerns associated with the use of embryonic stem cells as well as the problem of rejection, which occurs regularly in this type of induced cell. Of these factors, Oct4 and Sox2 are the “central” factors ones responsible for actually reprogramming the cell, while Klf4 and C-Myc simply amplify these effects, leading to the proliferation of pluripotent cells. Though Klf4 and C-Myc are not necessarily vital to this process (in fact, the latter is often substituted due to its oncogenic, or cancer-forming properties), it can be seen how they work best in conjunction [6]. Ideally, reprogramming will cause the Müller cells to return to an unspecialized, or progenitor, fate, which would allow differentiation into other damaged retinal neurons. Additionally, recent studies in zebrafish Müller glia have shown that Myc is upregulated in response to injury and is necessary for Müller glial proliferation [7]. Due to the natural abundance of two Yamanaka factors, Sox2 and Klf4, in Müller glia, the Müller tissues were transfected with L-Myc to induce proliferation. This factor is a relative of C-Myc but is less oncogenic, meaning it is less likely to result in excess cell division causing the development of cancerous tumors [8].
With these findings in mind, it was hypothesized that 1) L-Myc overexpression induces reprogramming and proliferation of Müller glial cells; 2) in tissues electroporated with L-Myc, Müller glia will be reprogrammed to a progenitor-like state.
MATERIALS AND METHODS.
The goal of these experiments was to test the capacity of the transcription factor L-Myc in causing reprogramming and proliferation of Müller glial cells in ex vivo retinal tissue cultures. Retinal tissue was harvested from transgenic mice (specifically the Rlbp1-CreER; RosaAi14 line), gathered from both adult males and females, with tissue from one eye constituting one sample and placed in culture. Only one eye per mouse was used per experimental condition. This culture method has characteristics of retinal injury and degeneration, which is the context in which it is ideal to promote regeneration. The L-Myc transfection was delivered via a novel submergence-free electroporation method using a column of agarose, which allows many short, high-voltage pulses of electric energy to bypass the cellular membrane and introduce target genes without having to submerge tissue in liquid, which can be damaging to tissues cultured at the air-liquid interface such as these [8].
A retinal progenitor state in adult murine Müller glia has been achieved with a variety of both vectors and introduced genes, including extracellular vesicles, RNA binding proteins, and even various G-protein coupled receptors (GPCRs) like α7 nAChR [9][10][11]. Ultimately, an approach similar to that of Nelson et al. [12] was decided– that is, a tissue culture model was established, and fluorescent imaging was utilized to determine levels of proliferation and reprogramming.
However, rather than introducing genes such as Ascl1 (as in Nelson et al.) –which can only produce certain cell types and is not robustly proliferative– achieving this progenitor state was accomplished via introduction of L-Myc, a variant of the Yamanaka factor C-Myc. These factors (Oct3/4, Sox2, C-Myc, and Klf4), used in addition to a fibroblast culture, can be used to create pluripotent stem cells, as shown in Takashihi et. al [13]. L-Myc is also more ideal to achieve proliferation, which is another significant indicator of tissue regeneration, as increased cell division contributes to healing of damaged tissue.
Murine subjects in this experiment were sourced from an in-lab transgenic line, specifically the Rlbp1-CreER; RosaAi14 line. This is a type of fluorescent imaging reporter and was used to confirm that Müller glial cells specifically were being returned to a progenitor state [5]. These were created via the Cre-Lox recombination method [14]. In this case, a stop codon was temporarily deleted, and a red fluorescent marker gene (TdTomato at the RosaAi14 locus) was added. Because of this Cre-mediated recombination process, affected Müller cells of these mice will show robust TdTomato expression and display a red glow when observed under fluorescence [15]. This allows for the mouse line to be used as a lineage tracing tool.
Data collection occurred over the course of 19 days, with the cells in culture being transfected with the selected genes 14 DEV. The samples were divided into two experimental groups: one control condition, which was electroporated with a PCMV-eGFP plasmid, and one experimental group transfected with a pCMV-LMYC-T2A-eGFP plasmid. These experimental groups were imaged two days post-transfection and seven days post-transfection. Due to the electroporation method being non-cell type specific, it was necessary for the measurement of proliferation to be accomplished using fluorescent markers. This was done with TdTomato and eGFP expression and antibody staining for Otx2 and BrdU. BrdU was introduced 24 hours before fixation.
Images were captured with a bifocal Structured Illuminescence Microscope (SIM) utilizing Apotome, and proliferation/ reprogramming was measured by quantifying fluorescence-positive cells using the 3D imaging software, Imaris [16]. Various images of the cultures were taken and used to produce large-scale renderings of the Müller cells. These renderings were used to quantify retinal tissue volume, proliferative cells, progenitor cells, cell type-specific markers and any overlap between markers. This is one method of validation. For example, if Otx2-expressing cells in the far red (Cy5) channel overlap with TdTomato cells in the red (Cy3) channel, which denotes Müller cells, and with eGFP cells marking L-Myc transfection, then it can be concluded that reprogramming is occurring in the Müller cells potentially due to the introduction of L-myc. Following Imaris quantification, a two-tailed student t-test was utilized for statistical analysis.
RESULTS.
The first aim of this study was to determine if L-Myc transfection would cause reprogramming, or the return of specialized retinal neurons to an unspecialized progenitor fate. In Figure 1, it is apparent that reprogramming is increased in the L-Myc transfected glia than in the control group. Notably, there are far more “triple” overlaps (eGFP, TdTom, and Otx2) evident than “double” (Otx2, TdTom), meaning that the reprogramming of Müller cells can almost certainly be attributed to the L-Myc transfection. This indicates that though some reprogramming of Müller cells can occur endogenously in response to injury at early time points, L-Myc transfection causes increased reprogramming.
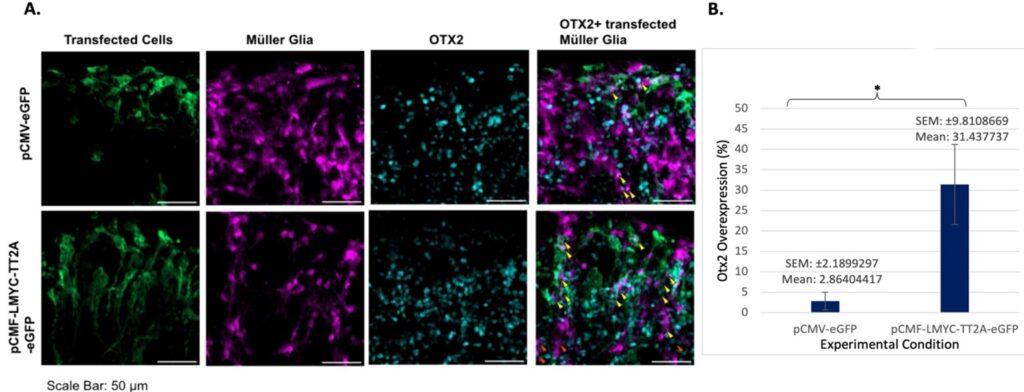
Reprogramming occurred in the seven-day sample (Figure 2) very similarly to the two-day sample, but at a heightened level, as evidenced by the greater number of overlaps. However, many of these overlaps were “double,” or simply TdTomato-expressing Müller cells and Otx2-expressing cells, meaning that the increase cannot be attributed with full confidence to L-Myc based off of this visualization (though it is believed retrospectively that the cells visible in some images, especially in the Otx2 samples, were actually overlapping in some instances where the cells appeared to be adjacent due to stitching inconsistencies).
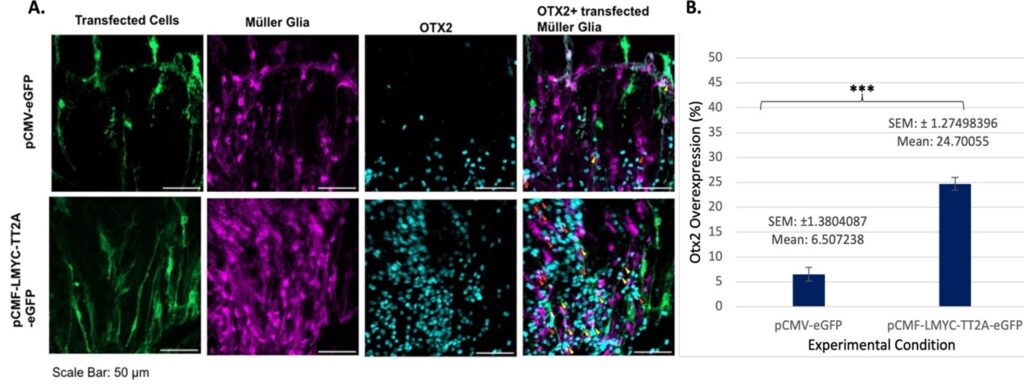
When considering later timepoints, it is important to keep in mind the length of time in culture: by 7 days post-transfection, the tissues become extremely thin, damaged, and degraded. This abundance of Otx2 markers present in the pCMV-LMYC-T2A-eGFP suggests that the L-Myc transfection may have played a role in this cellular reprogramming.
The second aim of this study was to determine if L-Myc transfection would increase the occurrence of cell proliferation, an important factor in the healing of damaged retinal tissue. BrdU, which represents proliferation, proved far more difficult to image than Otx2 due to the channel of visible light it was present in (in this study DAPI was utilized in the 359-461 nm range), and this incorporation is greater than the non-transfected samples.
It is important to keep in mind that many of these overlaps seen in Figure 3 were only “double”– simply BrdU and TdTom– but it is possible that there are more cells present that weren’t captured due to the fluorescent stain utilized. Though there are not a large amount of triple overlaps present in Figure 4, it is likely because the morphology of the imaged cells is slightly different than it is typically, likely due to the high amount of damage and degradation inherent in day seven samples.
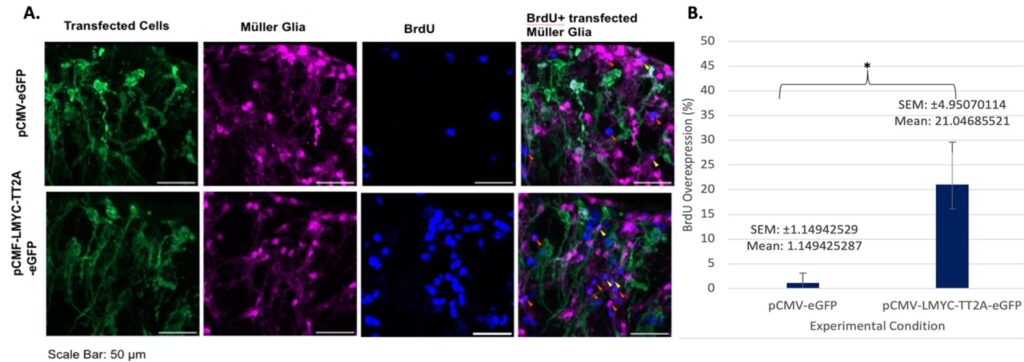
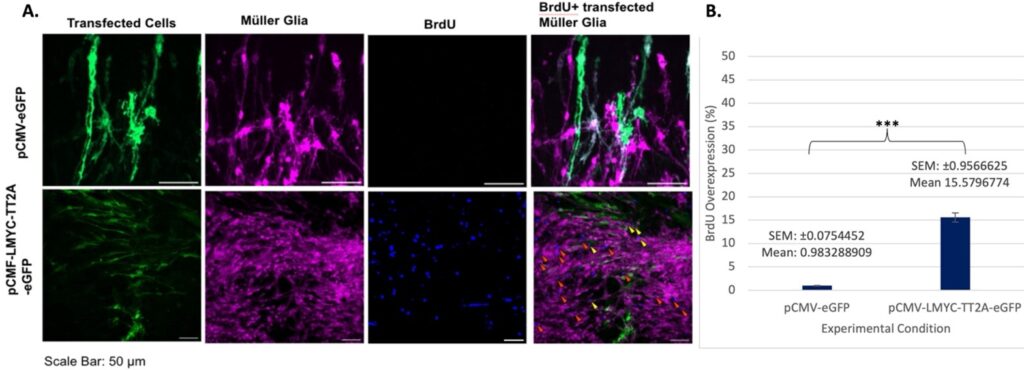
Overall, it was found that L-Myc transfection increases Otx2 expression and BrdU incorporation, indicating the occurrence of reprogramming and proliferation at early and later timepoints.
DISCUSSION.
Creating effective retinal therapies is highly difficult due to the complexity of photoreceptor layers and the variety of genetic and environmental causes of degenerative disease. Many of these treatments are disease-specific, applying only to the affected retinal cell type. Identifying genetic candidates that can cause regeneration of various different retinal cell types could pose implications for sourcing a treatment that is more universally applicable.
Utilizing electroporation and fluorescent markers, this paper sought to establish the viability of the transcription factor L-Myc in causing retinal regeneration by targeting proliferative and reprogramming capabilities of murine Müller glia. As shown by increased Otx2 expression and BrdU incorporation at both the two- and seven-day time points (with the later time points achieving far greater amounts of expression), L-Myc is a candidate for promoting regeneration. Results could be further refined with RNAseq and other methods of validation and tested in human retinal tissue.
The aim of this study was to determine if Müller glial cells can be induced to spontaneously proliferate or express progenitor cell markers using a promising genetic candidate. Identifying candidates to induce these changes in Müller glia could lead to genetic therapies to reverse vision loss in retinal degenerative diseases. Furthermore, the capability of MG cells to differentiate into other cell layer types means that if Müller cells can be generated, many diseases of the retina can potentially be cured. Though these studies are far from achieving a clinical impact, they will certainly lay the groundwork for this type of regeneration technology to be developed in the future. Ideally, this would work very similarly with almost complete vision restoration results. Overall, this study indicates that L-Myc transfection of Müller glia results in reprogramming and proliferation of this retinal cell type, highlighting L-Myc as a promising candidate for further regenerative studies.
While these findings are very exciting, it is important to take into account certain technical issues with the quantification process that may have led to inconsistencies. The Imaris 3D imaging software was utilized with the hope that it would lend greater accuracy to cell counts than 2D imaging software like Fiji, especially in respect to double-labeled cells, which prove challenging to quantify. While Imaris served this purpose, there were also unforeseen difficulties with its implementation. First, analysis of three fluorescent channels and three different cell morphologies meant that individual cell bodies were difficult to distinguish; this was especially true with TdTomato cells in the Cy3 channel denoting glia. Stitching errors for individual cells may have led to inaccurate quantifications–for example, false double- or triple-labeled cells, or conversely, seemingly adjacent cells that were in actuality overlapping. Additionally, the potential occurrence of color channel misalignments due to software error may have skewed counts. Moving forward, these issues must be rectified in order to place full confidence in the accuracy of these results.
ACKNOWLEDGMENTS.
Many thanks to members of the Levine-Fuhrmann Lab for their input and hospitality. Most especially, to Megan Stone for her mentorship and kindness and to Dr. Levine for enabling such an incredible experience!
REFERENCES.
- H. Vyawahare, P. Shinde, Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 14(9), e29583 (2022).
- S. Kobat, B. Turgut, Importance of Müller Cells. Beyoglu Eye Journal, 5(2), 59-63 (2020).
- Z. Jin, M. Gao, W. Deng, K. Wu, S. Sugita, M. Mandai, M. Takahashi, Stemming retinal regeneration with pluripotent stem cells, Progress in Retinal and Eye Research, 69, 38-56 (2019).
- G. Konar, “Overview of the Retina, Degenerative Eye Diseases, and Stem Cell Therapy,” Proceedings of 2023 academic lecture, Vanderbilt University.
- M. Stone, “MS F31 Research Strategy,” PhD research proposal, Vanderbilt University, Nashville (2023).
- X. Liu, J. Huang, T. Chen, Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Research 18, 1177–1189 (2008).
- M. Lee, J. Jui, A. Sahu, D. Goldman, Mycb and Mych stimulate Müller glial cell reprogramming and proliferation in the uninjured and injured zebrafish retina. Development 151(14), dev203062 (2024).
- M. Stone, “Reprogramming adult murine Müller glia in an ex vivo culture system,” thesis, Vanderbilt University, Nashville (2023).
- D. Didiano, Induction of a proliferative response in the zebrafish retina by injection of extracellular vesicles. Experimental Eye Research 200, 108254 (2020).
- J. Gaynes, The RNA Binding Protein Igf2bp1 Is Required for Zebrafish RGC Axon Outgrowth In Vivo. PLOS One 10(9), e0134751 (2015).
- M. Webster, Stimulation of Retinal Pigment Epithelium With an α7 nAChR Agonist Leads to Müller glia Dependent Neurogenesis in the Adult Mammalian Retina. Investigative Ophthalmology & Visual Science 60(2), 570-579 (2019).
- B. Nelson, Achaete-scute like 1 (Ascl1) is required for normal delta-like (DII) gene expression and notch signaling during retinal development. Developmental Dynamics 238(9), 2163-2178 (2009).
- K. Takashihi, S. Yamanaka, Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors, Cell, 126(4): 663-676 (2006).
- S. Bernal, M. Calaf, A. Adan, T. Solans, D. Valverde, C. Ayuso, M. Baiget, Evaluation of RLBP1 in 50 autosomal recessive retinitis pigmentosa and 4 retinitis punctata albescens Spanish families. Ophthalmic genetics, 22(1): 19–25, (2001).
- Jackson Laboratories, “B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J.” Description and Development of Transgenic Mice, (2023). https://www.jax.org/strain/007914. Accessed November 2024.
- Oxford Instruments, Imaris 10.2. Oxfordshire, England, UK (2024). https://www.oxinst.com/news/imaris-10.2-released. Accessed November 2024.
Posted by buchanle on Friday, June 20, 2025 in May 2025.
Tags: L-Myc, Müller glia, Proliferation, Reprogramming, Retinal Regeneration

