Passive solid-state adsorption of gaseous ozone by simple transition metal oxides
ABSTRACT
Whether in climate change, public health, or the synthesis of fuels and useful gasses, the capture of noxious heavier-than-air greenhouse gasses is a technology critical to human development in the coming century. In this paper, the passive gas adsorption abilities of simple transition-metal oxides were studied via the adsorption of ozone produced by gas-powered vehicle exhaust. The materials used were prepared via solid-state synthesis from carbonate precursors for nickel, copper, and zinc. Positive trends in reduction of ozone gas concentration in an experimental chamber were then observed. Several possible explanations for the relative efficacy of the different oxides investigated are presented, including partial charge interactions between O3 gas and the oxides used, and unpaired d-orbital electrons resulting from crystal orbital splitting after bonding.
INTRODUCTION.
Gas capture technology plays a pivotal role in addressing environmental challenges and promoting sustainable practices across various industries. Whether in industrial sectors, vehicle exhaust, or the active recapture of already-released greenhouse gasses, reducing the number of gaseous pollutants in the atmosphere is a critical aspect of mitigating climate change. By capturing and storing gasses in solid or liquid mediums[1], or repurposing them for other applications, gas capture technology contributes significantly to reducing the effects of climate change and minimizing the overall carbon footprint of many industries and technologies[2].
In the realm of air quality management, gas capture finds application in controlling toxic emissions from various sources, including industrial facilities and transportation. Through these systems, harmful pollutant gasses like nitrogen and sulfur oxides can be effectively captured and removed before being released into the atmosphere[3]. High concentrations of these gasses pose a direct threat to public health, with one of the single largest contributors to this issue being ozone gas. Individually, high tropospheric ozone (O3) concentration is estimated to be responsible for over 365,000 deaths annually[4]. Although gasoline combustion in vehicles doesn’t directly produce ozone, the reaction of other unstable gaseous products in the air creates a substantial amount of the gas[5]. Thus, O3 originating from vehicle exhaust is a large contributor in deaths related to air pollution, and the focus of this paper’s investigations.
Existing solutions to localized and global pollution based on gas capture show promise, with several such methods in active development. However, these methods have proven to be expensive, hard to scale, and difficult to implement outside of specifically engineered ultra-high temperature or pressure systems[6]. Many solutions employ carbon capture technology directly at manufacturing sites; while effective at reducing emissions from large manufacturing and synthesis industries, these methods of gas capture lack implementation due to high cost and a fair degree of liability risk[6].
Simple (single cation) metal oxides were chosen as the primary gas-capture medium to be tested for this investigation. These species were targeted due to their extremely low cost and ease of synthesis, making experimental testing and analysis simple. The hypothesis that the transition metal species within the oxide will influence the adsorptive properties of the material is presented. A null hypothesis that these oxides will display no passive gas-capture properties is also presented.
MATERIALS AND METHODS.
Material Synthesis, Characterization, and Treatment.
Carbonates of copper(II), nickel, and zinc (Lab Alley, unknown ‘lab-grade’ purity) were thermally decomposed in crucibles (100-gram capacity, ceramic silica) over an open flame to yield their respective oxides. Prior to use, crucibles were thoroughly sterilized by heat exposure. 25 grams of copper(II), nickel, and zinc carbonate were then weighed (digital mass scale, Eophorus) and transferred to their respective crucibles, which were covered with a ceramic lid during synthesis. Carbonates were reacted by heating the bottom of each crucible with a propane torch set to around 750°C. Heating continued for about ten minutes, until a full color change of the material could be observed- dark green for NiO, black for CuO (Figure 1, bottom panel). For ZnO, since no color change occurs during the reaction, the material was heated for an extended period of 30 minutes.

End products were stored in a dry, airtight container to prevent passivation of any active surface area by exposure to water vapor or other ambient gasses prone to sorption. Following a heating cycle to free trapped gasses, the oxides were weighed again. If any variance in mass from original pre-trial measurements was still present, enough material was prepared and added to return the sample to its initial mass for the next trial.
Experimental Chamber Design.
To create the experimental chamber, an electrochemical ozone gas sensor (VQP, detection range of 0-5 ppm) was first adhered to the bottom of a clear polypropylene container with the display facing onto the plastic. One end of a plastic tube was inserted into this opening, then securely sealed and insulated; the other end of the tubing was adhered to the muffler of the vehicle and was similarly attached. Finally, the lid was fitted on the container, which was then placed lid-down on the ground with the face of the sensor facing upwards (Figure 1, top panel). All trials were conducted at an ambient temperature of 16.85°C and standard ambient pressure. Conditions inside the chamber were at similar pressure, but at a temperature range of 70°C to 85°C (varying by trial).
Methods and Conditions of Gas Adsorption Measurement.
Prior to each trial, the vehicle (2012 Subaru Forester, 2.5L 4-cylinder) was allowed to run undisturbed for at least five minutes to ensure consistent motor function. After this warm-up period, the experimental chamber was attached to the tailpipe of the car, with ozone gas running through the tube into the container (Figure 1). The motor was then left to run until ozone concentration readings were relatively consistent – 土0.010 ppm optimally, although this wasn’t consistently achieved. At this point, the container was quickly lifted, and an uncovered metal oxide sample was introduced; the timer was started as soon as the oxide contacted the gas in the container. Ozone concentration levels were periodically observed at the time the sample was introduced, then at 5 one-minute intervals from this point forward.
RESULTS.
Broadly, each oxide tested appeared to reduce the local concentration of ozone gas in the chamber. When left without a sample, ozone levels remained constant, dropping only 0.02 ppm from 0.40 immediately after sealing to around 0.38 after the five-minute observation period (Table 1). Exposure to each species of oxide was associated with an immediate drop in gas levels; from zero to one minute after sealing, initial trials containing copper(II) oxide, nickel oxide, and zinc oxide saw decreases in concentration of O3 of 31.58%, 36.11%, and 27.78%, respectively (raw data provided in Supporting Information).
| Table 1. Absolute and average % change in ozone concentration, % change normalized by oxide mass, and absolute change (ppm) in ozone normalized by oxide mass. | |||||||
| No sample | CuO Trial 1 | CuO Trial 2 | NiO Trial 1 | NiO Trial 2 | ZnO Trial 1 | ZnO Trial 2 | |
| Percent change in ozone levels | -5.00% | -31.58% | -30.77% | -36.11% | -40.54% | -27.78% | -33.33% |
| Mean percent change in ozone levels | -5.00% | -31.18% (CuO) | -38.33% (NiO) | -30.56% (ZnO) | |||
| Percent change ozone per gram oxide mass | 0.000% | -1.460% | -1.470% | -1.680% | -1.890% | -1.360% | -1.630% |
| Absolute change in ppm ozone per gram oxide mass | 0.000 ppm | 5.56E-2 ppm | 5.55E-2 ppm | 6.37E-2 ppm | 7.35E-2 ppm | 4.44E-2 ppm | 5.33E-2 ppm |
Relative Efficacy of Oxide Species.
While ozone measurements were considerably lower than normal conditions in each trial conducted with a metal oxide, reductions in ozone concentration were consistently different for some materials, despite relatively minor variances in mass of oxide exposed by species. To allow a more even frame of reference based on the amount of metal oxide used, data from Table 1 were normalized to percent change per gram of oxide. Overall, trials containing nickel oxide manifested the greatest absolute and normalized percent changes in gas levels, with % decreases in ppm ozone of 36.11% in trial 1 and 40.54% in trial 2, and % decreases per gram oxide of 1.679% and 1.886%. Comparing similar figures from the first and second trials involving CuO and ZnO, we see percent decreases of 31.58%/30.77% and relative percent decreases of 1.462%/1.471% for copper; and 27.78%/33.33% and 1.362%/1.634% in the same measurements for zinc (Table 1). By interpreting decreases in ozone concentration as a measure of adsorption efficacy, we can infer that nickel oxide was the most effective at sequestering O3. Interpretation becomes more difficult with copper(II) and zinc oxide as both species proved to be of relatively similar efficacies; mean percent reduction in ppm ozone of CuO was only ~0.540%.
DISCUSSION.
Several potential mechanisms are proposed to explain the trends in ozone absorptivity across the metal oxide species investigated in this paper. These mechanisms are predicted based on known chemical and electronic properties, periodic trends, and existing literature.
Dipole-Dipole Interactions.
The main mechanism proposed to explain the adsorptive effect of the synthesized metal oxides on ozone is simple partial-charge interactions. Broadly, the dipole moment from ozone’s molecular geometry is significant enough to cause interaction with charged solid mediums; when exposed to specifically designed carbon catalysts, gaseous ozone molecules were chemically adsorbed using hydroxide pathways, and subsequently used as a secondary catalyst for carbon-carbon double bond formation[7]. This example highlights how dipole-dipole interactions can play a strong role in gas adsorption of simple transition-metal oxide materials.
All oxides investigated had electronegativity differences large enough to qualify them as polar covalent compounds, with ΔEN values of 1.53, 1.52, and 1.79 for NiO, CuO, and ZnO, respectively. Thus, when in non-passivized form, each molecule would’ve had a significant dipole moment, with partial positive and negative charges on the metal and oxygen respectively. Similarly, ozone gas also manifests a dipole moment. While a Lewis dot representation of the molecule would show two different geometries for ozone – with each state having one oxygen double bonded and the other with a lone partial negative charge – the molecule still functions as one with a partial charge on each atom, this time with δ+ on the central oxygen, and δ–1/δ–2 on the bonded oxygens. In combination, the interaction between these partial charges may have been enough to pull the less stable ozone molecules towards the surface of the transition-metal oxide particles (Figure 2A), causing the downward trend in gaseous ozone readings observed through all trials that contained a metal oxide.
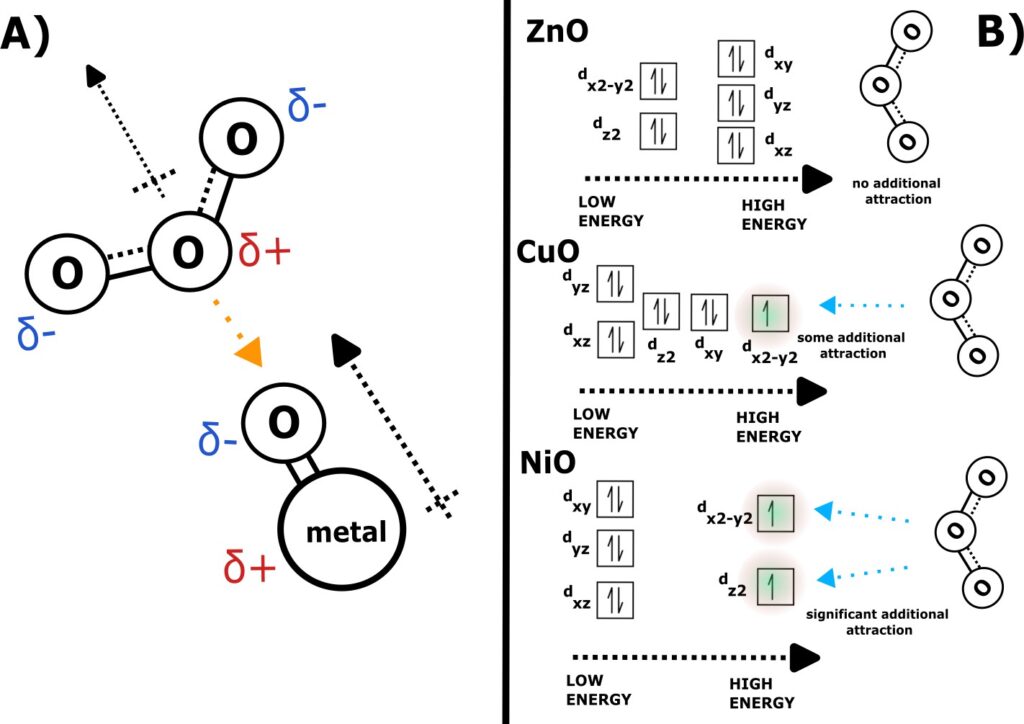
Effect of D-Orbital Electron Configuration.
While well-defined dipole-dipole interactions could explain why transition-metal oxides are broadly able to sequester polar gasses like ozone, they aren’t sufficient to explain the large internal difference in efficacy between the oxides investigated. Seeing as how nickel, copper(II), and zinc oxides were all associated with notably different mean percent ozone decreases through multiple trials involving each (Table 1), there logically must be a second mechanism outside of pure dipole interactions affecting adsorption efficacy.
It is proposed that this mechanism is additional attractive forces originating from the number and presence of unpaired valence electrons in bonded transition metals. Based on the observed optical properties of the nickel oxide and copper(II) oxide investigated and the literature around these materials, the Ni2+ is assumed to be an octahedral site within its crystal structure, while the Cu2+ is assumed to sit in a square planar site.
In these structures, Ni and Cu would’ve both have had all the electron(s) in their 4s orbitals contributing to a covalent bond with oxygen. The resulting d-orbital electron configuration for each transition-metal ion is outlined in Figure 2B; nickel oxide is left with two unpaired electrons in dz2 and dx2-y2 owing to orbital splitting from its octahedral crystal structure[8], while copper(II) oxide is left with a single unpaired electron in dx2-y2 due to its respective square planar structure[9]. Zinc has enough valence electrons in its metallic form to avoid having to contribute d-orbital electrons to bonding and is thus left with a full set of d-orbital electrons paired. Experimentally, these differences in electron configuration may explain why nickel and copper(II) oxide were relatively more effective at attracting the unevenly distributed electrons in ozone than zinc, and thus were more effective adsorption agents with higher percent differences in ozone concentration in each trial. The lone electrons present in the d-orbital of each metal would’ve exerted an attractive force on ozone molecules that supplemented the existing attractive dipole interactions between O3 and each metal oxide, leading to the internal gaps in % change ppm between the three species investigated. From Table S1, we see that trials involving NiO saw a mean 38.33% decrease in ppm ozone, compared to 31.18% in CuO and 30.56% in ZnO.
While the gap in efficacy from Cu to Zn isn’t as significant as the gap between Ni and Cu, this could be explained by simple electronegativity trends; with a cation EN of 1.65 versus copper’s 1.90, zinc oxide would’ve had a moderately larger ΔEN between its constituents than copper(II) oxide. Thus, the zinc oxide samples observed would’ve had a more significant dipole moment, making up for some of the attractive force ZnO lacked relative to CuO due to its full d-orbital.
CONCLUSION.
Overall, a series of simple transition-metal oxides were synthesized and tested for their O3 adsorption properties. In addition to dipole interactions as a driving mechanism for why these materials adsorb the polar O molecule, an interesting trend emerged when evaluated the electronic structure of each material: with an increase in unpaired valence electrons in the d-orbitals of the transition-metal, an increase in O3 adsorption was observed. To further validate the role of electron structure in this trend, additional metals with varying numbers of d-orbital electrons or different crystalline structures could be investigated, like titanium, silver, or tungsten. Additional measurements should be performed to elucidate the contribution of surface area in the materials investigated. Particularly, X-ray photoelectron spectroscopy (XPS)[10] and X-ray absorption Near Edge Structure could be used to confirm the electronic structure of the transition-metal ions. In addition, Brunauer–Emmett–Teller (BET) and dynamic light scattering measurements should be performed to garner surface area and particle size of the materials used, respectively[11].
In future investigation, the experimental methods of this study could be refined to reduce measurement error, improve the precision of findings, and allow for more conclusive identification of relevant electronic trends. A more advanced design of the experimental chamber could allow O3 gas to be flushed through the chamber, ensuring a consistent atmosphere, with a wider dish being used to increase area of exposure for any oxide used; this design would also employ more stringent sealing measures and pure ozone gas, alleviating some issues with gas purity/leakage present in this experiment. The period of observation could also be extended to better outline the behavior of O3 concentration past 5 minutes, revealing whether the adsorption investigated behaves linearly until a sharp cutoff at saturation or continues to function logistically as time goes on. Finally, concentrations of other gasses majorly present – namely CO2, H2O, and nitrogen oxides – could be observed in parallel to determine whether some competitive effect exists between these gasses and the O3 gas primarily being monitored. Broadly, future iterations of this project should aim to use more advanced equipment and facilities to better control confounding variables in the ozone adsorption process.
Although this research focused experimentally on the surface-level capture of ozone gas, similar principles and technology could be extended to other areas of study. Notably, the investigation of technology able to sequester other prevalent polar gas pollutants, like sulfur and formaldehyde, would have significant application. Generating a better understanding of the electronic properties that allow certain polar mediums to be used in gas capture is critical to expanding and improving on existing technologies, and continuing the investigations of d-orbital electron interactions outlined in this paper would be a key component of such expansion.
ACKNOWLEDGMENTS.
Thank you to Dr. Nicholas Grundish for helping me understand the basics of crystal field theory despite being extremely busy on the other side of the country.
SUPPORTING INFORMATION.
Additional supporting information includes:
Table S1: Raw gas concentration data for each trial.
REFERENCES.
- M. Ramdin, State-of-the-art of CO2 Capture with Ionic liquids. Ind. Eng. Chem. Res.51, 24, 8149–8177 (2012)
- S. Paltsev, Hard-to-Abate Sectors: The role of industrial carbon capture and storage (CCS) in emission mitigation. Applied Energy.300, 117322 (2021)
- J. Bell, Adsorption of Carbon Dioxide, Water Vapor, Nitrogen, and Sulfur Dioxide on Activated Carbon for Capture from Flue Gases: Competitive Adsorption and Selectivity Aspects. Energy Fuels.35, 8102–8116 (2021)
- C. Rasmussen, Local Lockdowns Brought Fast Global Ozone Reductions, NASA Finds. Jet Propulsion Laboratory. (2021), (available at https://www.nasa.gov/centers-and-facilities/jpl/local-lockdowns-brought-fast-global-ozone-reductions-nasa-finds/)
- National Research Council, Ozone-Forming Potential of Reformulated Gasoline (National Academies Press, 1999)
- X. Wang, Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Frontiers in Energy Research.8, 44-45 (2020)
- X. Luo, The Adsorption of Ozone on the Solid Catalyst Surface and the Catalytic Reaction Mechanism for Organic Components. ChemistrySelect.5, 15092 (2020)
- R. Sanjeev, A simple rule of thumb for the explanation of d-orbital splitting in complexes. SciELO.31, 4 (2020)
- J. Börgel, Transition Metal d-Orbital Splitting Diagrams: An Updated Educational Resource for Square Planar Transition Metal Complexes. J. Chem. Educ.93, 118-121 (2016)
- M. Aziz, “Chapter 5 – X-Ray Photoelectron Spectroscopy (XPS)” in Membrane Characterization (Elsevier, 2017), pp. 81-93
- M. Naderi, “Chapter Fourteen – Surface Area: Brunauer–Emmett–Teller (BET)” in Progress in Filtration and Separation (Academic Press, 2015), pp. 585-608
Posted by buchanle on Tuesday, April 30, 2024 in May 2024.
Tags: crystal field theory, green technology, Greenhouse gas adsorption, inorganic materials, intermolecular forces

