Optimization of Chlorophytum comosum Growth Rate Using Arduino
ABSTRACT
More accessible computer technology has broadened technological developments for far-ranging humanitarian purposes, including food production. Previous research has involved using devices made of easily accessible hardware and software to monitor plant conditions, and it is important to determine if such monitoring can improve outcomes such as plant growth. This research explored the efficacy of such devices in improving plant growth. First, a device was built that detected environmental temperature, humidity, and soil moisture. Caretaking decisions for Chlorophytum comosum plants equipped with the device were based on the device-computed values of these variables. The growth of the device-equipped plants was compared to that of control plants lacking the device. Caretaking decisions of control plants were based on visual and tactile soil criteria. The heights of plants in both groups were measured frequently during two trials. It was found that both the total plant growth (Trial I: p=0.844, Trial II: p=0.58) and percentage growth (Trial I: p=0.81, Trial II: p=0.582) were not significantly impacted by use of the device. These results indicate the importance of testing computer technology to ensure it improves agriculture before implementation, as automation may not be superior to human intervention.
INTRODUCTION.
As the world’s population has rapidly increased, it has become necessary to expand the world’s capacity for producing food, medicinal plants, and other agricultural goods. Technologies necessary to achieving this goal commonly involve automated devices that sense values of different environmental factors and control a device, such as a water pump, based on these values. Such technology can allow agriculture to become more efficient and less labor intensive, but can introduce flaws, since these systems involve no human intervention, meaning there is often no way of addressing malfunctions.
The primary goal of previous research has been to create systems that can be expanded and improved upon to influence agriculture on a larger scale. Most of this research has focused on small-scale automated agricultural systems that irrigate small groups of plants. Notable research has utilized Arduino technology to build systems which automatically irrigate plants when their soil moisture, which is read by sensors, falls below a set threshold [1]. This research has recently expanded to monitoring several environmental values, such as temperature and humidity, in conjunction with soil moisture, to provide further insight into the plant’s environment and enhance irrigation techniques [2,3]. Other studies have examined the efficacy of systems that involve having a researcher treat plants based on values obtained from sensors, rather than automating this treatment [4,5]. These systems allow for more human control over the conditions of plants and eliminate some risk of malfunction, such as the risk of mechanical parts that interact directly with plants degrading or breaking, by removing the physical interference of technology with the plants. Human interference also allows other types of malfunction, such as faulty sensor readings, to be addressed, as human interference necessitates that someone review incoming sensor data.
While previous research has made great advances in automated agricultural technology, the question remains whether such technology is an improvement upon current methods for monitoring plant conditions. Previous research has made the assumption that monitoring or irrigation aided by technology will improve plants’ health and growth when compared to plants grown without technological aid. To test this assumption, the present study developed an Arduino monitoring device that allows for a human user to observe the conditions of plants and treat them based on the specific values of these conditions. A testing group of Chlorophytum comosum plants were intermittently assisted by the device over a period of several weeks and these plants’ heights were compared to those of a control group of plants grown during this same time without device assistance. A comparison in growth between the two groups tested the hypothesis that technological aid would significantly increase the growth of plants in the testing group. Arduino technology was chosen to facilitate this research because of its intuitive and easily constructed hardware and software as well as the prevalence of Arduino technology in previous research into agricultural monitoring and automation.
MATERIALS AND METHODS.
Section A: Device Configuration.
AI: Device Wiring.
All six of the plants in the testing and control groups were obtained from a local gardening store, where they had been partially grown. They were kept on a level surface in indirect sunlight throughout the duration of the experiment.
An Arduino device was configured to measure the temperature, humidity, and soil moisture of the growth environments of the three testing group Chlorophytum comosum plants. The Arduino device was equipped with two sensors: a DHT11 sensor from Adafruit Industries in New York City that measured temperature and humidity, and a Seeed Grove sensor that measured soil moisture. The soil moisture sensor had initially been meant to be compatible with a microcontroller similar to Arduino, but with slightly different wiring, so the ends of the sensor’s wires were cut to be compatible with a standard Arduino Uno microcontroller and breadboard. Both sensors were connected to a 3.3V power source and ground. The DHT11 sensor was connected to pin 13, a digital input pin, and the soil moisture sensor was connected to A1, an analog input pin. The raw sensor data was transcribed to the Arduino IDE’s Serial Monitor. The data was read and the humidity and soil moisture values were used to decide on caretaking actions, while temperature values were used to ensure that the temperature of the environment remained within a suitable range (21°-32°C) for Chlorophytum comosum growth.
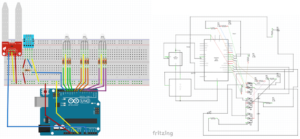
The device was also equipped with three RGB LEDs, which were each connected to 3.3 volts of power and to three digital output pins. Each LED changed color depending on the value of either temperature, humidity, or soil moisture, with color changes being controlled by digital output pins. The output of each pin was generated based on collected values from the associated sensor, so that each LED changed color based on the current value of its associated parameter. The complete wiring of the device is displayed in Figure 1.
Figure 1. The Arduino device was equipped with a DHT11 sensor that was connected to digital input pin 13 and a Seeed Grove soil moisture sensor that was connected to analog input pin A1. Three RGB LEDs were each connected to three digital output pins.
AII: Device Code.
The code for the device performed the following four functions: First, it imported the libraries necessary to control each sensor. Second, it initialized each of the needed pins on the Arduino board as an Input or Output (I/O) pin so that the pins meant to take in sensor data would be preset to take in data and the pins meant to output voltage to the LEDs would be preset to output voltage. Third, it used input pins to retrieve data from each sensor. Finally, it controlled the output to the LEDs based on the readings from each sensor.
All code was written in the Arduino Integrated Development Environment (Version 1.8.16). The primary purpose of the program was the retrieval of data from the soil moisture, temperature, and humidity sensors. The program also used this data to control the LEDs. The part of the program that read DHT11 data was adapted from code written by M. Schwartz [6] and utilized commands from the official DHT11 library [7]. Soil moisture data was read using code included in a Mouser Electronics guide detailing how to use the sensor [8].
Section B: Plant Maintenance and Data Collection.
BI: Trial I Testing Group Plant Treatment.
Three times per week for three weeks (June 15 – July 6), each of the testing group plants (n=3) were temporarily equipped with the sensor device, which returned values indicating their soil moisture, humidity, and temperature. Based on this data, the environmental factors of these plants were altered according to the following protocol:
- If the soil moisture sensor returned a value of less than 250, the plant from which the value was obtained was watered. 250 was listed by the soil moisture sensor developer as an appropriate value for the slightly moist soil best for Chlorophytum comosum growth [8].
- If the humidity percentage fell below 50%, the plant was misted, as a humidity percentage of over 50% is appropriate for common houseplants such as Chlorophytum comosum when they are mature, but not yet flowering [9].
After each testing group plant had been equipped with the device, the plants which required watering or misting were treated according to the listed conditions and were then equipped with the device a second time. After every plant that required treatment had been watered and/or misted and monitored again, the height of each plant was measured using the distance from the tip of the tallest leaf, which was held so that its base formed a 90° angle with the soil, to the nearest point on the rim of the plant’s pot. This measurement process was repeated with the second and third tallest leaves of each plant, and an average of the three heights from each collection period was used in data analysis for each plant.
BII: Trial I Control Group Plant Treatment.
At the same times each week that the testing group plants were monitored, the control group plants (n=3) were monitored using non-technological practices. Decisions to water each plant were made based on a visual and tactile protocol that reflected common home gardening practices. An outline of this protocol can be found online in Table S1 of this article’s supplemental information.
After each control group plant received its proper treatment, the same process for measuring the height used on the testing group plants was repeated for three leaves of each control group plant.
Section C: Soil Moisture Calibration and Changes to Trial II.
Trial II took place for an additional 3 weeks (September 8 – October 3). Some minor changes were made from Trial I to Trial II.
During Trial I, it was difficult to gauge soil moisture using the corresponding sensor due to the lack of units specified within the sensor’s readings. To mend this, calibration of the soil moisture sensor was carried out by testing the sensor in several samples of soil which had a known soil moisture percentage. To prepare the soil samples for calibration, dry soil was weighed and, using soil weight, the amount of water needed to create a specific percentage (0-20%) of soil moisture was calculated and added to the dry soil. Once the water had distributed throughout the soil, the soil moisture sensor was submerged in the soil, and the value it returned for each soil moisture solution was recorded. Raw data from this calibration is listed in Table S2 of the supporting information for this article.
The soil moisture sensor generally returned a value between 350 and 400 for soil moistures between 10% and 15%, which are optimal for the growth of Chlorophytum comosum [10]. Based on the soil moisture sensor’s calibration data, the soil moisture value at which the testing group plants would be watered was changed from 250 to 350. Additionally, the schedule for monitoring and watering the plants in both groups was altered so that monitoring and watering only occurred twice per week and height measurement only occurred once per week. This change was implemented as only negligible differences in plant height were observed between measurements in Trial I.
Section D: Statistics.
The data for each trial is normalized with a base measurement of 0 for the first date of the trial. Two-tailed t-tests were run on the height data collected from each plant and the average total growth of the plants in each treatment was compared.
The average percentage growth of each plant during Trials I and II was calculated using the following formula, with Hf representing the plant’s final height, as determined by averaging the heights of its three tallest leaves, and Hb representing the initial height at the beginning of the trial, which was determined in the same way:
Percentage Growth = (Hf-Hb)/Hb
RESULTS.
Total Plant Growth.
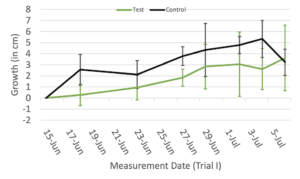
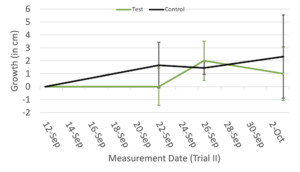
In Trial I (Figure 2A), the average growth of the control group with no device assistance remained higher than that of the device-assisted testing group for most of the duration of the trial, with the testing group only beginning to overtake the control group at the end of the trial. This trend was, however, not significant (p = 0.844). Similarly to Trial I, in Trial II (Figure 2B), the average growth of the control group still generally remained higher than that of the testing group. This trend was still not significant (p = 0.58).
Figure 2. The average total growth of each plant in the testing group (assisted by device) and control group (no device assistance) between each measurement date during Trial I (A) and Trial II (B). Green error bars represent the standard deviation of the testing group, and gray error bars represent the standard deviation of the control group.
Percentage Growth.
To account for smaller plants that may have only grown slightly, and to further corroborate the results presented in the previous section, percentage growth was calculated in addition to total growth.
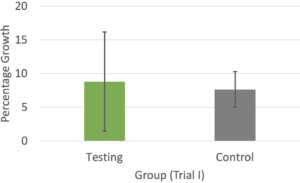
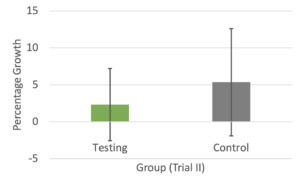
In Trial I (Figure 3A), the percentage growth of the testing group was slightly greater than that of the control group, but this difference was not significant (p = 0.81). In Trial II (Figure 3B), the control group had a slightly greater percentage growth. This difference was also not significant (p = 0.582).
Figure 3. The average percentage growth of the plants in the testing and control groups on the last measurement dates of Trial I (A) and Trial II (B). Error bars represent standard deviation.
DISCUSSION.
This project hypothesized that use of a device that monitored temperature, humidity, and soil moisture would significantly increase the overall growth of the testing group plants. This would support the general notion that the use of technology in agriculture improves output. The collected data, however, rejects this hypothesis. Figure 2 indicates that, by the end of both trials, usage of the configured device did not significantly improve the height of plants in the testing group when compared to those in the control group (p>0.05 for both trials). Figure 3 further indicates that usage of the device did not significantly improve percentage growth amongst the testing group plants (p>0.05 for both trials). The difference in both total growth and percentage growth between the groups was more significant in Trial II, with p-values of 0.58 and 0.582, which are less than Trial I’s p-values of 0.844 and 0.81. These results indicate that technology may not always improve output, which is important, as the idea that technology consistently improves output is the basis for many engineering projects that work towards the automation of agriculture [1-5].
This research reveals that it is not indiscriminately true that computer technology improves agriculture. It would be best, considering that this assumption is not guaranteed to be true, to rigorously test all devices which are meant to improve agricultural growth or yield to ensure that they are effective in these areas before they are used for large scale agriculture.
This research was limited by the components used to build the device. The components were chosen with the intention of building a low-cost, affordable monitoring device. Some of the sensors that were used were not made for extensive, lasting use, which, at times, led them to return erratic measurements. These erratic measurements produced data with high variability. The accuracy of the collected data might have been hindered by such hardware.
In the future, it would be valuable to replicate this research using a plant with more well-documented environmental needs, or with more specific needs, so that caretaking of the plant could be more precisely informed by sensor measurements. The spider plant was chosen partially due to its resilience in different environments, as this resilience made the plant easier to keep alive and test consistently. This resilience, however, also lessened the impact of the environmental changes that were made based on the device’s readings, which made the collected data less reflective of the adequacy of these changes. It would also be interesting to test if a wireless device would be more effective in improving plant growth or to subject the device built in this research to additional tests that could indicate its effectiveness in accurately monitoring its environment.
For this research, a device was built that monitored the temperature, humidity, and soil moisture of a testing group of Chlorophytum comosum plants. This device was used to monitor and inform the caretaking of these testing group plants, while a control group of plants was monitored and cared for without use of computer technology. Periodically, the height of each plant in both the testing and control groups was measured, and a comparison of the measurements of the plants in both groups indicated that the use of computer technology did not significantly affect plant growth.
ACKNOWLEDGMENTS.
Thank you to the School for Science and Math at Vanderbilt for funding and facilitating this research. Thank you to Dr. Haag and Dr. Eeds for being wonderful mentors and supporting this research.
SUPPORTING INFORMATION.
Table S1: Watering protocol for control group plants
Table S2: The composition and returned soil moisture value of each solution
REFERENCES.
- Prasojo, A. Maseleno, O. Tanane, N. Shahu, Design of Automatic Watering System Based on Arduino. J. Robot. Control JRC. 1, 59-63 (2020).
- Putjaika, S. Phusae, A. Chen-Im, P. Phunchongharn, K. Akkarajitsakul, A control system in an intelligent farming by using arduino technology. 2016 Fifth ICT International Student Project Conference (ICT-ISPC). 53-56 (2016).
- Selvaraj R. S., I.M., A. Keerthika, Arduino based Smart Irrigation System for Home Gardening. ICICT. 6, 1284–1288 (2021).
- Puengsungwan, K. Jirasereeamornkul, Internet of Things (IoTs) based hydroponic lettuce farming with solar panels. ICPEI, 86–89 (2019).
- Sihombing, N. A. Karina, J. T. Tarigan, M. I. Syarif, Automated hydroponics nutrition plants systems using arduino uno microcontroller based on android. J. Phys. Conf. Ser. 2 (2018).
- Schwartz, WiFi Weather Station. Adafruit Learning System (2013).
- DHT sensor library. Adafruit Industries (2021).
- Grove – Moisture Sensor User Manual. Seeed Studio (2015).
- What is the Right Humidity for Growing Plants Indoors?. Safer brand.
- F. J. Veihmeyer, A. H. Hendrickson, Soil Moisture in Relation to
Plant Growth. Annu. Rev. Plant Physiol. 1, 285–304 (1950).
Posted by John Lee on Saturday, May 7, 2022 in May 2022.
Tags: Arduino, Chlorophytum comosum, Spider plant