Measuring the Effect of IL-8 and fMLP on Neutrophil Chemokinesis Using Artificial Intelligence
ABSTRACT
Neutrophils are essential components of the innate immune system and play a pivotal role in the body’s response to infections by phagocytosing (eating) pathogenic organisms and releasing chemokines (signaling molecules) critical for inflammation. Neutrophils move to sites of inflammation by following gradients of chemokines, which can also stimulate changes in cell motility, shape, and phagocytosis. Using artificial intelligence (AI) to track neutrophils in in vitro chemokinesis assays, this study seeks to compare the effects of two chemokines, N-formyl-methionine-leucine-phenylalanine (fMLP) and Interleukin-8 (IL-8), on neutrophil chemokinesis when exposed individually and in combination. Our investigations revealed that neutrophils exposed to IL-8 alone exhibited higher levels of motility than when exposed to fMLP alone. However, when exposed to both IL-8 and fMLP in combination, neutrophils exhibited intermediate levels of motility (lower levels than IL-8 alone but greater levels than fMLP alone). These findings shed light on the complex interplay of chemokines in neutrophil motility and emphasize the potential application of AI cell tracking to explore the nuances of neutrophil motility in response to various signaling molecules.
INTRODUCTION.
Neutrophils, often referred to as polymorphonuclear leukocytes (PMNs), constitute 60% of circulating leukocytes (white blood cells) and serve as the first line of defense against infectious agents, playing a critical role in inflammatory responses (Nathan, 2006). Neutrophils are highly responsive to pathogen and host signals that cause cell activation and may affect cell shape and motility. In response to signaling molecules called chemokines induced by infection or tissue damage, neutrophils adhere to the endothelium and exit the bloodstream to migrate toward the site of injury during a process called extravasation (Schmidt et al., 2011). Neutrophils were exposed to two types of chemokines in this study: N-formyl-methionine-leucine-phenylalanine (fMLP) and Interleukin-8 (IL-8). Host-derived IL-8, is secreted by nearby macrophages, mast cells, and myeloid cells and serves as a mediator for inflammation and plays an intermediary role for leukocyte migration. This enables neutrophils to reach the vicinity of infection through a chemotactic gradient (Blake & Allen, 1988). Once in the vicinity, neutrophils follow gradients of chemoattractants, such as fMLP, which are released by bacteria into their environment as a byproduct of protein synthesis (Gauthier et al., 2007). At the site of infection, fMLP serves as an end-target attractant, leading neutrophils along the gradient to the source of infection or injury, such as bacteria (Metzemaekers et al., 2020). This mechanism ensures a highly efficient and precise immune response, allowing neutrophils to swiftly and accurately execute their immune defense functions while minimizing collateral damage to neighboring cells.
fMLP has a single receptor known as Formyl Peptide Receptor (FPR), while IL-8 can bind to CXCR1 (IL-8 receptor type 1) and CXCR2 (IL-8 receptor type 2). All three receptors are members of the seven-transmembrane helix receptor family. Once bound to their ligand, these receptors transmit their signals to heterotrimeric G proteins, which activate downstream pathways leading to rapid cytoskeletal rearrangements and chemotaxis (Campbell et al., 1997). Although these chemokine receptors share similar structures and signaling axes, neutrophils exhibit profound variations in their responses to fMLP, IL-8, and their combination, including distinctions in structural phenotype and chemotactic differences (Hirsch et al., 2000). Using a chemokinetic setting rather than a chemotactic, we can gain deeper insights into the nuanced differences in cell motility when exposed to stimuli alone and in combination.
When investigating the effects of movement stimuli on cell motility, researchers typically employ two primary methods: chemotaxis and chemokinesis. Chemotaxis is defined as the directional cell movement of cells towards concentration gradients of solubilized attractants, whereas chemokinesis is defined as random cell movement in the absence of chemoattractant gradients (Liu, Z. et al., 2004). Previously, researchers have used manual methods to examine the impact of fMLP and IL-8 on neutrophil shape, activation, and motility in the context of chemotaxis (Hattenkofer et al., 2018), and have observed preferential neutrophil migration toward end-target chemoattractants like fMLP, even in the presence of high concentrations of host-derived intermediary chemoattractants such as IL-8 (Heit et al., 2002). Recent advancements in AI and machine learning have made it possible to conduct high-throughput and thorough analysis of neutrophil motility using AI image analysis (Vobugari, N. et al., 2022). This can be particularly valuable for scrutinizing subtle distinctions in motility patterns within a chemokinetic framework. In this study, we used AI image analysis to track cells and explore the hypothesis that neutrophil exposure to a combination of chemokinetic agents, such as IL-8 and fMLP, will lead to significantly increased neutrophil motility than the motility observed with either of these agents alone.
MATERIALS AND METHODS.
Microscopy.
All experiments were imaged on a Nikon TI-2 epifluorescent microscope using a 40x air objective with a 0.6 numerical aperture. At this setting, one pixel is consistent with 6.25µm. Additionally, all experiments were performed with an aligned correction collar to account for air/glass/water interfaces. An Okolab enclosure around the TI-2 maintained the apparatus at 37°C, pH 7.4, and 5% CO2 for the duration of the experiments to closely resemble conditions in the human body. BF images were captured over 45 minutes on a 45-second interval using the NIS Elements program during live cell imaging experiments. The migration chamber was fixed on the microscope table and six distinct locations (three for each experiment) were electronically monitored every 45 seconds, creating 61 frames per timelapse video.
Migration Analysis.
Neutrophil migration was tracked by entering timelapse videos into the Ibidi Fast Chemotaxis FastTrack AI Image Analysis, where recognition of individual cells was conducted based on a deep learning algorithm. Cell boundaries were determined through BF images, and the cell centers were determined through a series of the center of mass calculations based on cell borders.
Outputted tracking data were extracted, cleaned, and separated from FastTrack AI using programs written in Python language in PyCharm IDE (JetBrains). Appendix A presents the AI-tracked data once extracted, cleaned, and sorted (see discussion for specifics). Once determined that the extracted, cleaned, and sorted data remained consistent with the cell’s actual movement as represented by Fast Track AI, the data was entered into MATLAB (MathWorks) computational software to be analyzed.
To conduct more comprehensive analysis of each chemokine’s effect, neutrophils were separated into three categories: non-motile, motile, and locomotive cells. Cutoff values for separating neutrophils into distinct motility categories were established by visually choosing ten non-motile, motile, and locomotive cells and averaging the accumulated and Euclidean distances for each category. The average distances for non-motile and motile cells were used to define the cutoff between non-motile and motile, while the average distances for motile and locomotive cells were used to establish the cutoff between motile and locomotive cells.
Two measures of distance traveled were used in each experiment: accumulated distance and Euclidian distance. The accumulated distance was calculated as shown in Eq. 1, where i represents the frame number from the time-lapse, xi corresponds to frame i’s x coordinate pixel, and yi corresponds to frame i’s y coordinate pixel.
Euclidean distance was calculated as shown in Eq. 2, where x60 represents the x coordinate pixel of frame 60 (the last frame), and x1 represents the x coordinate pixel of frame 0 (the first frame). Both equations were derived from the distance formula and were multiplied by 6.25, which was necessary because the settings on our microscope indicated that each pixel on each frame of the timelapse corresponded to 6.25 micrometers (µm) on the microscope stage.
\[\sum_{i=1}^{60}6.25 \times \sqrt{\left(x_i-x_{i-1}\right)^2+\left(y_i-y_{i-1}\right)^2}\tag{1}\]
\[6.25 \times \sqrt{\left(x_{60}-x_0\right)^2+\left(y_{60}-y_0\right)^2}\tag{2}\]
Statistics.
Data was pooled from a minimum of at least 5 different donors, with the total degrees of freedom for each experimental condition exceeding 1,000. Analysis of variance was performed using MATLAB and Excel. The null hypothesis was rejected if P was less than .05.
Additional Materials and Methods.
Check SI Document for specific reagents, cell preparation and substrate preparation.
RESULTS.
Effects of Chemokines on Total Accumulated and Euclidean Distance.
In order to determine neutrophil responses to chemoattractants in combination and alone, four distinct experimental conditions were established: Unstimulated (control), IL-8 (7nM), fMLP (100nM), and fMLP + IL-8 (7nM and 100nM, respectively). Table 1 shows the criterion of classification for non-motile, motile, and locomotive cells in terms of accumulated distance (the total distance traveled by the neutrophil) and Euclidian distance (the magnitude of displacement between the start and end point of the neutrophil). Non-motile cells were cells that exhibited minimal displacement and often do not migrate independently. Their movements were often detected due to external factors, such as microscope stage adjustments during observation and erroneous classification of movement by AI image tracking, primarily because of alterations in cell shape rather than actual cell migration. Motile cells were characterized as cells that exhibited motion but did not traverse significant distances from their initial positions during the observation period. Locomotive cells were defined as cells that both demonstrated a more directed movement and traveled substantially from their original location. This categorization helps us explore the heterogeneity within neutrophil populations and understand how different subsets of neutrophils contribute to immune responses and inflammation under varying conditions. It allows for a more detailed assessment of their behavior, contributing to a comprehensive analysis of their role in host defense and inflammatory processes.
| Table 1. Cutoff values (μm) for non-motile, motile, and locomotive categories. “A” denotes the accumulated distance covered by the neutrophil, while “E” represents the Euclidean distance traveled by the neutrophil. | |||
| Non-motile | Motile | Locomotive | |
| Accumulated | A ≤ 32 | 32 < A ≤ 96 | 96 < A |
| Euclidean | E ≤ 3 | 3 < E ≤ 8 | 8 < E |
As depicted in Figure 1A, we conducted a comparison of non-motile, motile, and locomotive neutrophil proportions based on total accumulated distance. Our analysis revealed that neutrophils exposed to IL-8 in isolation demonstrated the highest percentage of locomotive neutrophils, followed by fMLP + IL-8, fMLP alone, and unstimulated neutrophils. Conversely, unstimulated cells exhibited the highest percentage of non-motile neutrophils, followed by fMLP, IL-8, and fMLP + IL-8. In Figure 1B, we compared proportions based on total Euclidean distance. Our analysis revealed that neutrophils with IL-8 exposure resulted in the highest percentage of locomotive neutrophils, followed by fMLP + IL-8, fMLP alone, and unstimulated neutrophils. Similar to the accumulated distance population, unstimulated neutrophils displayed the highest percentage of non-motile neutrophils, followed by fMLP, IL-8, and fMLP + IL-8.
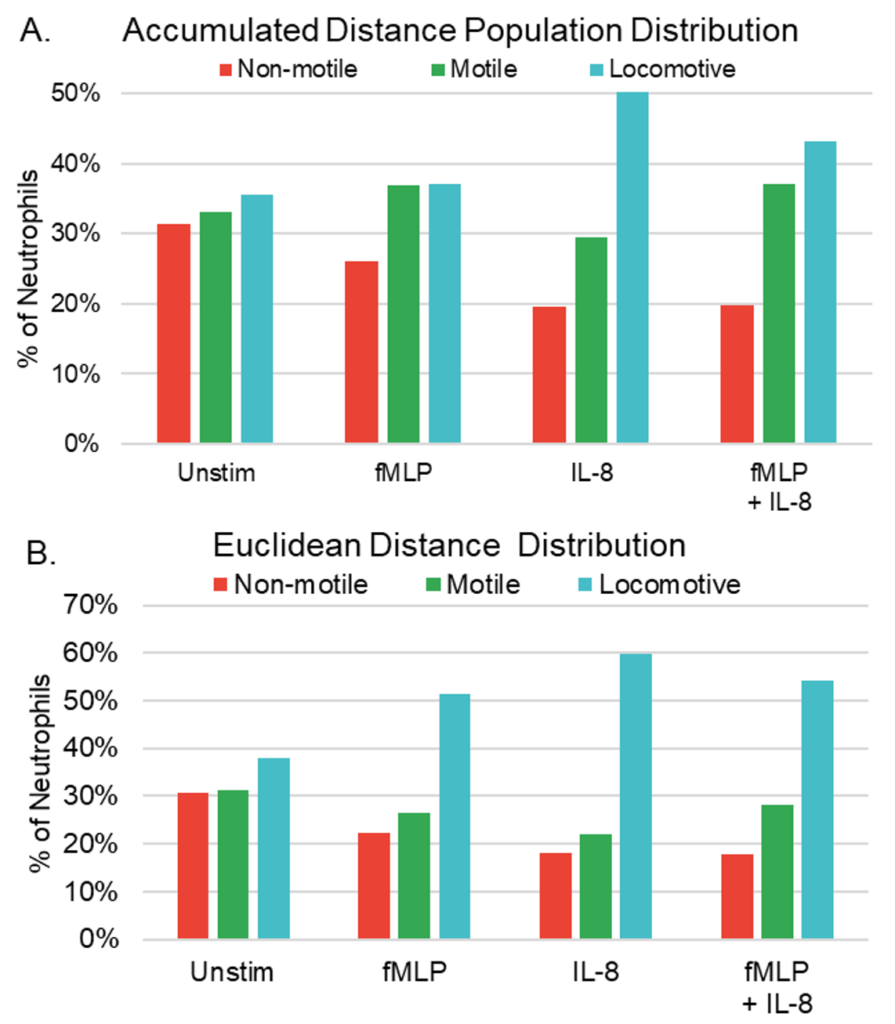
The observed trend where neutrophils exposed to fMLP + IL-8 exhibited a lower proportion of locomotive cells compared to those exposed to IL-8 alone, yet a higher proportion than those exposed to fMLP alone, suggests that neutrophils simultaneously exposed to fMLP + IL-8 may exhibit characteristics influenced by both chemoattractants.
Effects of Chemokines on Directness.
The chemotactic index (CI) is a quantitative measurement describing the directionality of cell migration to the direction of a gradients, also called forward migration index (Hu, Y., 2003). CI is calculated by Eq. 3. When the CI equals one, it signifies that the Euclidian distance equals the accumulated distance and the cell trajectory follows a linear path, as illustrated in Appendix B. In contrast, a CI ratio less than 1 indicates that the Euclidian distance is less than the accumulated distance, and the cell did not move in a straight line, rather, the cell traveled in a less direct path to its destination.
To determine if neutrophils exposed to fMLP + IL-8 may simultaneously display characteristics of both chemoattractants, we identified the CI of neutrophils exposed to our four conditions. Migration was found to be influenced by the specific chemokinetic stimulant to which the neutrophils were exposed, as shown in Figure 2. In each experimental condition, scatter plots were used to depict the motility of individual cells concerning their accumulated and Euclidean distances. Each condition’s regression line was utilized to represent the mean CI for each experimental condition.
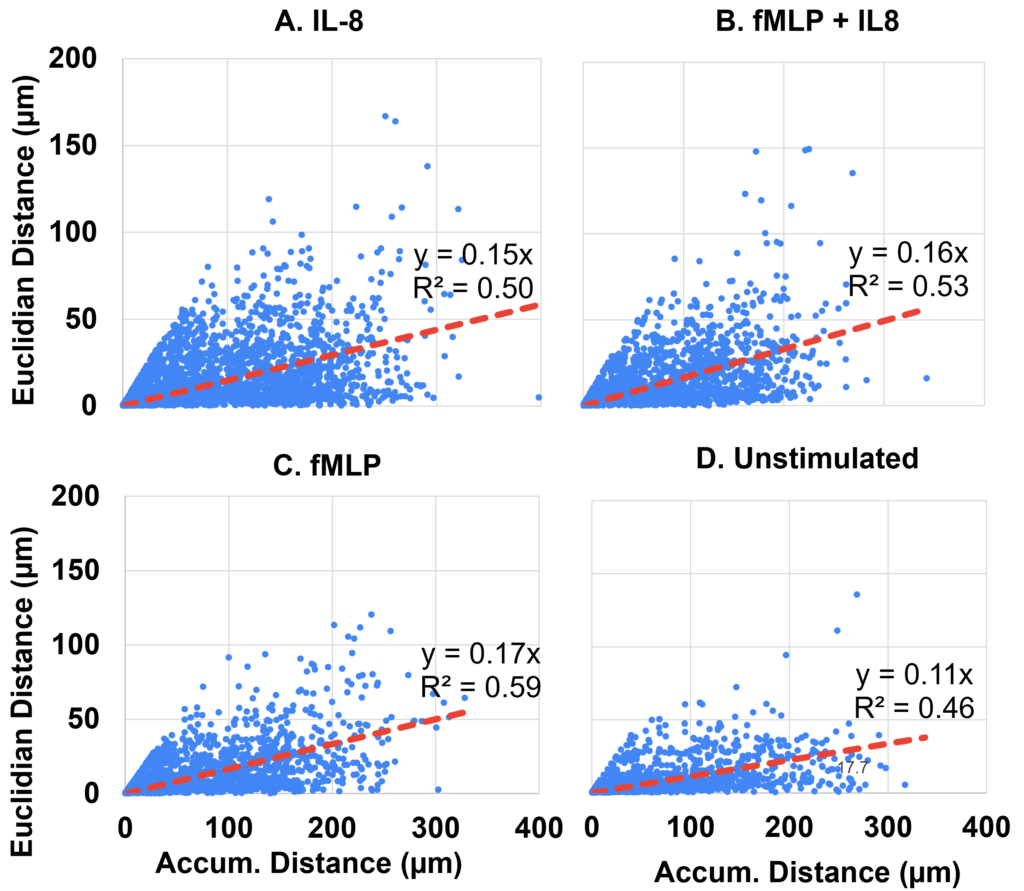
In terms of directness, fMLP expressed the highest CI, indicating that neutrophils exposed to fMLP alone had the most direct trajectory (shown by the highest slope of Figure 2). We also observed by gross observation that neutrophils stimulated with fMLP not only have an increased directness in movement, but also incur other phenotypic changes such as increased surface area known as flattening or “pancaking,” similar to phenotypes expressed by Neutrophils that have adhered to stiffer substrates (Oakes et al., 2009). Neutrophils exposed to fMLP + IL-8 were the second most direct, followed by IL-8 alone, and unstimulated neutrophils, suggesting that neutrophils exposed to two chemokines may display a combination of characteristics from both chemokines. Although the slopes of the stimulated conditions were similar, they all differed significantly from the slope of the unstimulated neutrophils, which we also observed through gross observation (data not shown).
\[CI = \frac{Euclidian Distance}{Accumulate Distance}\tag{3}\]
Effects of Chemokines on Mean Accumulated and Euclidean Distance.
Experiments demonstrated that different chemoattractants impacted mean neutrophil accumulated and Euclidean movement (summarized in Table 2). IL-8 demonstrated the highest average motility. fMLP + IL-8 showed lower motility than IL-8, but a higher motility than fMLP and unstimulated group. Neutrophil mean accumulated distance, shown in Figure 3A, and mean Euclidean distance, shown in Figure 3B, varied based on the chemoattractant. IL-8 exhibited the greatest distance traveled, followed by fMLP + IL-8. Conversely, fMLP and unstimulated neutrophils, exhibited the least amount of accumulated distance and showed no significant difference in accumulated distance from each other. For mean Euclidean distance, IL-8 exhibited the greatest distance traveled, followed by fMLP + IL-8, then fMLP and unstimulated neutrophils, with the control (unstimulated) neutrophils showing a significant decrease in Euclidean distance than those exposed to chemoattractants. Interquartile range (IQR) 1, 2, and 3 also varied substantially among experimental groups in terms of both accumulated, shown in Figure 3C, and Euclidean distance, shown in Figure 3D. Exemplary bright field (BF) images of tracked neutrophils and their varying levels of motility are shown in Appendix C.
| Table 2. Mean accumulated and Euclidean distance | ||||
| Average | Unstimulated | fMLP | IL-8 | fMLP+IL-8 |
| Accumulated | 80.0 μm | 83.2 μm | 104.0 μm*** | 89.7 μm*** |
| Euclidean | 10.3 μm | 15.0 μm*** | 17.7 μm*** | 16.6 μm*** |
| Degrees of Freedom | 1,196 | 1,505 | 2,185 | 1,935 |
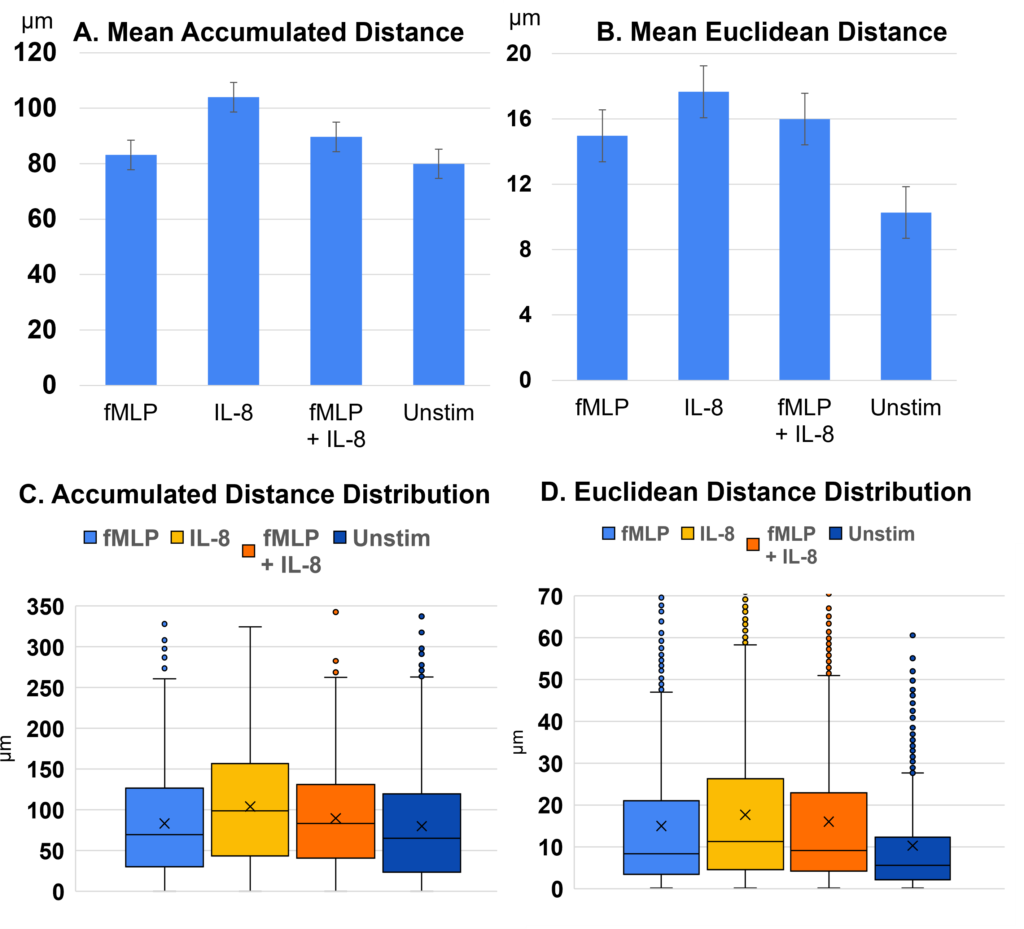
The observed trend that neutrophils exposed to fMLP + IL-8 averaged the two stimulants’ effects on motility, once again, suggests that neutrophils simultaneously exposed to fMLP + IL-8 may exhibit characteristics influenced by both chemoattractants.
Using a T-test, the p-value for each condition is compared to the control (the unstimulated condition). *** indicate P < .001. As assessed through accumulated and Euclidean distance analyses, neutrophils exhibited the following decrease in motility in this order across experimental conditions: IL-8, fMLP + IL-8, fMLP, and Unstimulated experiments, with IL-8 resulting in the highest motility.
DISCUSSION.
Using AI image analysis of migration assays offers several key advantages; it enables the analysis of a larger volume of cells in a consistent manner, and improves time- and resource- efficiencies, allowing researchers to obtain more reliable and larger samples. However, it is important to consider the current limitations of Fast Track AI’s chemotaxis analysis software and conducting chemokinesis on a chemotaxis software.
One issue encountered in AI cell tracking was prematurely terminating the tracking of a cell and subsequently re-tracking it as a new cell. To address this problem, a minimum track duration threshold of 30 frames was implemented, effectively reducing occurrences of the AI mistakenly tracking a single cell multiple times in shorter instances. In addition, this step corrected cases where the AI erroneously identified other entities, such as blank areas, shadows, and inorganic matter, as neutrophils for short periods of time. Tracks associated with non-motile inorganic matter (such as particulate matter and precipitation formed during hydrophilic treatment of wells) were specifically addressed by removing tracks that did not exceed an empirically assigned minimum cutoff value of 10µm in accumulated distances. Finally, to address the problem of AI also misidentifying cells that intersect during migration (often leading to tracks jumping from cell to cell causing disruption in inaccurate representation of neutrophil motility), tracks that reported a change of 24 µm of Euclidian distance within 1 frame were removed from the data, as it was determined unlikely for neutrophils to cover that distance within 45 seconds, the time between each timelapse frame. This problem was also addressed by limiting the number of neutrophils in each Ibidi µ-Slide 2 Well chamber to 250,00 neutrophils to minimize neutrophil clustering and collision and thus limiting opportunities for the AI to misinterpret the cell boundaries/position.
To ensure each neutrophil’s movement was accurately represented after data cleaning and MATLAB analysis, an additional confirmational procedure was run for each time-lapse field of view. Python libraries such as Pyautogui (used to take screenshots of the timelapse video), cv2 (used for image processing), numpy (used to create and manipulate arrays), and random (used to generate random numbers) were used. Contrasting shades (bright and dark) were used to determine four random cells that were chosen from 4 different quadrants of the field of view. The track numbers corresponding to these randomly selected cells were then extracted from the tracking data provided by Fast Track AI. Those 4 tracks were then run through the same data cleansing algorithm to ensure that the cells weren’t inaccurately tracked by the AI. Track shapes, accumulated distances, and Euclidean distances were then analyzed using the Chemotaxis and Migration tool (Ibidi) to ensure their accurate representation of the respective cell’s movement, as depicted in the time-lapse video, even after data cleaning and isolation (see Appendix A). In addition, accumulated distance, Euclidean distance, and population distribution of the randomly chosen cells were also checked to ensure that the final MATLAB-derived data was feasible and coherent within the context of the experimental condition. After taking further steps to mitigate inconsistencies with AI tracking, we believe our methods can be used to produce data that accurately represents the motility of neutrophils in any chemokinetic environment.
Our results show that neutrophils exposed to IL-8 showed greater motility in terms of accumulated and Euclidean distance when compared to those exposed to fMLP. In addition, neutrophils exhibited reduced motility when exposed to fMLP+IL-8 compared to IL-8 alone, but greater motility when exposed to fMLP. This reduced motility could be associated with receptor saturation. The receptors for these molecules, Formyl Peptide Receptor (FPR), CXCR1 (IL-8 receptor type 1), and CXCR2 (IL-8 receptor type 2), may be fully saturated when exposed to fMLP+IL-8, limiting the ability of neutrophils to respond effectively to either stimulus (Campbell, Foxman, & But, 1997). However, this reduced motility could also be an effect of neutrophils simultaneously exhibiting properties of both chemoattractants. By gross observation, we noticed neutrophils with a flattened morphology tended to migrate in more direct, yet less motile trajectories. The reduced motility could be neutrophils simultaneously responding to fMLP, causing a more flattened and direct movement but less motile distance traveled, while also responding to IL-8, leading to a rounded and more motile but less direct phenotype.
Future work includes titrating IL-8 and fMLP to different concentrations and comparing motility to test saturation, observing neutrophil migration with CXCR1 and CXCR2 blocked receptors when exposed to fMLP, and testing neutrophil motility for IL-8, fMLP, and IL-8 and fMLP in combination on varying gel stiffnesses.
SUPPORTING INFORMATION.
Supporting Information includes detailed Materials and Methods, BF image comparing cleaned and tracked data, diagram comparing the ratio of Euclidian to Accumulated distance for direct migration and indirect migration, and sample BF images comparing IL-8, fMLP, fMLP + IL-8, and unstimulated cell tracks.
ACKNOWLEDGMENTS.
Thank you to Ian Wong Madison Smith, Jayna T, and Vivian Griffin for the helpful conversations and informative resources.
REFERENCES.
- Nathan, C., Neutrophils and immunity: Challenges and opportunities. Nature Reviews Immunology, 6(3), 173-182 (2006).
- Schmidt, E. P et. al., On, around, and through: neutrophil-endothelial interactions in innate immunity, Physiology, 26(5), 173-182 (2011).
- Blake, D. R., & Allen, R., Inflammation: Basic Principles and Clinical Correlates, Annals of the Rheumatic Diseases, 47(9), 792 (1988).
- Gauthier, F., Fortin, A., Bergeron, Y., Dumas, C., Champagne, E., & Bergeron, M. G., Differential Contribution of Bacterial N-Formyl-Methionyl-Leucyl- Phenylalanine and Host-Derived CXC Chemokines to Neutrophil Infiltration into Pulmonary Alveoli during Murine Pneumococcal Pneumonia. Infection and Immunity, 75(11), 5361-5367 (2007).
- Metzemaekers, M., Gouwy, M., & Proost, P., Neutrophil chemoattractant receptors in health and disease: Double-edged swords, Cellular & Molecular Immunology, 17(5), 433-450 (2020).
- Campbell, J. J., Foxman, E. F., & Butcher, E. C., Chemoattractant receptor cross-talk as a regulatory mechanism in leukocyte adhesion and migration, European journal of immunology, 27(10), 2571–2578 (1997).
- Hirsch, E. et. al., Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation, Science, 287(5455), 1049–1053 (2000).
- Liu, Z., & Klominek, J., Chemotaxis and chemokinesis of malignant mesothelioma cells to multiple growth factors. Anticancer research, 24(3a), (2004).
- Hattenkofer, M. et. al., Time course of chemotaxis and chemokinesis of neutrophils following stimulation with IL-8 or fMLP. European Journal of Inflammation, (2018).
- Oakes, P. W. et. al., Neutrophil morphology and migration are affected by substrate elasticity, 1387-1395. Blood, 114(7), (2009)
- Vobugari, N. et. al., Advancements in Oncology with Artificial Intelligence-A Review Article. Cancers, 14(5), 1349, (2022).
- Hu, Y., Becker, M. L., & Willits, R. K., Quantification of cell migration: metrics selection to model application. Frontiers in cell and developmental biology, 11, 1155882, (2023).
Posted by buchanle on Tuesday, April 30, 2024 in May 2024.
Tags: Artificial Intelligence, Cell migration, Chemokinesis, Immunology, Neutrophils

