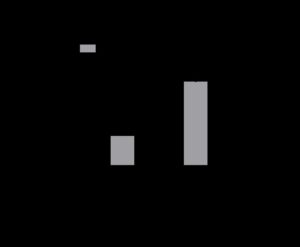Maintenance and expansion of myeloid progenitor cells in 3D culture
ABSTRACT
Myelodysplastic syndrome (MDS) is a condition where blood cells in the bone marrow do not mature, causing a halt in production of blood cells that can lead to total bone marrow failure and leukemia. Myeloid cells come from the common myeloid progenitor lineage of multipotential hematopoietic stem cells and have a strong tendency to differentiate quickly into megakaryocytes, erythrocytes, mast cells, or myeloblasts. Studying myeloid progenitor cells may be crucial for understanding the pathology of MDS and to develop treatments against MDS and other primary bone diseases. However, since 2D in vitro models do not represent the spatial organization of cells and cell interactions, it was hypothesized that a 3D model of myeloid progenitor cell culture could have an advantage to researching the problems leading to MDS. To study this, we isolated hematopoietic stem cells (HSCs) from the bone marrow of mice and cultured them in 3D scaffolds mimicking human trabecular bone. After 10 days in culture, live cells were counted with trypan blue and mRNA was extracted for gene expression analysis. Cells grown in vertebral body scaffolds had highest proliferation compared to proximal tibia and mandible scaffolds, while cells grown in mandible scaffolds had highest gene expression for genes associated with a stem cell state. Therefore, our results show that HSCs grown in bone mimicking 3D culture can proliferate and maintain a stem-like state.
INTRODUCTION.
Myelodysplastic Syndrome (MDS), often referred to as a bone marrow failure disorder and previously known as preleukemia, is a condition in which the bone marrow stops producing healthy blood cells. Often diagnosed with a rank of risk from the International Prognostic Scoring System (IPSS), this syndrome often leads to complete bone marrow failure and even leukemia, a cancer that lives in the body’s blood-forming tissues that causes blood cells to grow abnormally and restrict the growth of healthy blood cells [1,2]. In MDS, most of the concern is placed on the hematopoietic stem cells (HSCs) because HSCs are the multipotent stem cells from which myeloid progenitor cells and blood cells are formed [3]. For MDS patients, progression of the disease occurs when the progenitor cells lack the ability to fully differentiate, resulting in an overabundance of defective and non-functioning cells and leading to a progressive depletion of healthy blood cells [4]. It is believed that HSCs do not differentiate because of the apoptotic nature of myelodysplastic syndrome’s progression [5,6]. Finding a way to replace these non-functioning HSCs with healthy ones could be the key to treating MDS patients.
Culturing stem cells such as HSCs and maintaining them in a pluripotent state is challenging. Successful cultures require mimicking a more in vivo microenvironment [7]. Current culturing protocols for stem cells involve the addition of growth factors, co-culture with support cells, and pseudo 3D culture in Matrigel. However, little research has been done looking at a bone-mimicking 3D environment for culturing and maintaining stem cells. Culturing HSCs in 3D culture could give us a better opportunity to observe the biological processes that they undergo when committing to which specific cell types they will become and to see if there is a way to control their differentiation. 3D culture could also improve the expansion of myeloid progenitor cells in culture because it would allow the cells to attach themselves to the scaffolds similar to how they may grow in vivo.
Our group has designed 3D scaffolds mimicking the microarchitecture and chemical components of trabecular bone and may provide a more accurate physiological microenvironment for culturing HSCs. It has been previously shown by our group and by others that cells grown in these bone-mimicking 3D scaffolds represent the tumor microenvironment more accurately and allow cells to have stronger physiologically relevant responses [8]. Therefore, we hypothesize that 3D culture of stem cells in bone mimetic 3D scaffolds will allow the expansion and maintenance of myeloid progenitor cells. This will provide a way to more accurately study the differentiation processes of HSCs and allow us a better understanding on the progression of MDS.
MATERIALS AND METHODS.
Fabrication of 3D Scaffolds.
To simulate the different structure and trabecular architecture of bone, 3D scaffold molds were modeled after human trabecular bone at Vertebral Body (VB), Proximal Tibia (PT), and Mandible (M) sites [8]. To make the bone mimicking 3D scaffolds, a mixture of nanohydroxyapatite (nHA) and polyurethane (PUR) was injected onto the top of the molds and sucked through with a vacuum. Afterwards, the filled molds were placed in a vacuum oven at 50֯ C to solidify overnight. The next day, the molds were dissolved in 70% acetone and placed in a water bath for a few minutes until all mold was gone. The scaffolds then went into the oven overnight again to harden until they could be sterilized before being infused with cells in media.
Cell Collection and Isolation.
To retrieve uninfected common myeloid progenitor cells, bone marrow was extracted from the hindlimbs of BALB/c mice by another member of the laboratory using centrifugation. The primitive HSCs were isolated using a Miltenyi Biotec Direct Lineage Cell Depletion Kit. Cells were resuspended in 80µL of buffer plus 20 µL of Direct Lineage Cell Depletion Antibody Cocktail against CD5, CD11b, CD45R (B220), Anti-Gr-1 (Ly6G/C), 7-4, Ter-119 and incubated for 10 minutes on ice. After incubating, the cell suspension was magnetically separated by running through a LS magnetic separating column (Miltenyi). The column was washed with 3×3 mL of buffer to collect the remaining unlabeled cells. The negative fraction (Lin- HSCs) was collected and used for all subsequent experiments.
Tissue Culture.
To assess proliferation and gene expression of Lin- HSCs, cells were plated on 3D scaffolds and treated with Stemline II Hematopoietic Stem Cell expansion media supplemented with 10ng/mL interleukin3, macrophage colony stimulating factor, granulocyte-macrophage colony stimulating factor, and 100ng/mL stem cell factor (Common Myeloid Progenitor, CMP, media). To start, scaffolds were sterilized with a 70% ethanol wash and then UV sterilization for 15 min. Scaffolds were coated with 4ug/mL Fibronectin (Invitrogen) to aid in cell attachment. Cells were seeded into scaffolds at a concentration of 50,000 cells in 30uL media. After 1-hour incubation, 200uL media was added per well. To grow out the common myeloid progenitor cell population from the Lin- HSCs, cultures were given CMP media. Half of the spent media was refreshed every three days before harvesting on the tenth day to be counted and analyzed.
Proliferation Assay.
After 10 days in culture, the cells were washed in PBS buffer and incubated with Accutase for 10 minutes to remove cells from the scaffolds. Cells were then washed in PBS and counted. Live cell numbers were collected by mixing the cell suspension with trypan blue and counting on a TC20 automated cell counter. Expansion of cell populations were assessed by comparing the number of cells collected after 10 days in culture to the starting cell concentration to show overall population growth of the cells.
qPCR.
To screen the populations of cells grown out from the Lin- HSCs, gene expression analysis was performed for a panel of genes specific to the primitive cell state, the common myeloid progenitor state, and a more committed myeloid state. Briefly, cells grown on the 3D scaffolds for 10 days were lysed with TRIzol and mRNA was purified using RNeasy kit (Qiagen) following manufacturer’s protocol. cDNA was made from 1ug RNA using Quanta Biosciences qScript cDNA supermix. Taqman qPCR was performed for the following genes: GAPDH, CSF1, CSF2, IL6, ITGB1, ITGAM, TGFbr2, ATP6v0D (Table 1). Relative gene expression was calculated using the ddCT method.
The genes analyzed, their description, and their expression are listed as follows: ITGB1 (Integrin beta 1)- Hematopoietic Stem Cells; ATP6v0D (ATPase H+ Transporting V0 subunit d1)- Hematopoietic Stem Cells, CMPs Monocytes; TGFbr2 (Transforming growth factor beta receptor 2)- Hematopoietic Stem Cells, CMPs; CSF-1 (Macrophage Colony stimulating factor)- CMPs, Monocytes; CSF-2 (Granulocyte-Macrophage Colony stimulating factor)- Common Myeloid Progenitors; IL-6 (Interleukin 6)- Monocytes.
Statistical Analysis.
All statistics were performed using the GraphPad Prism software. One- or two-way ANOVA was used to calculate statistical differences. Graphs report the mean and standard error. P < 0.05 is considered statistically significant with n = 3 for all experiments.
RESULTS.
To assess proliferation of Lin- HSCs in 3D culture, cells were collected after 10 days and counted with trypan blue. As shown in Figure 1, after ten days in culture, roughly a third of the cells grown in the Proximal Tibia scaffolds had died and the cells grown in the Vertebral Body had almost doubled in number (1.7-fold increase, p<0.01). No cells were able to be detected from the mandible scaffolds (N.D. = not detected). The cells grown in the Vertebral Body had the highest proliferation over all the other scaffold types.
Figure 1. Primitive stem cells were cultured in CMP media for 10 days on 3D scaffolds mimicking the proximal tibia, vertebral body, and mandible. Live cells were counted using trypan blue. Cells grown on Vertebral Body 3D scaffolds exhibited significant expansion with a 1.7-fold increase in cell number after 10 days. Cells grown on Proximal Tibia 3D scaffolds showed no significant expansion of cells after 10 days in culture, and we were unable to collect cells from the Mandible 3D scaffolds. n=3 scaffolds. **p<0.01, 2way ANOVA with Sidak’s multiple comparisons test.
In order to verify which sub-populations of cells were expanding out from the Lin- HSCs seeded into the 3D scaffolds, gene expression analysis was performed for a panel of genes specific to the primitive cell state (ITGB1, ATP6v0D, TGFbr2), the common myeloid progenitor state (CSF-1, CSF-2), and a more committed myeloid state (IL-6, ITGAM). As shown in Figure 2, cells grown in the mandible scaffolds showed the highest overall mRNA expression, with a statistically significant increase for all genes except for ATP6v0D and CSF-1 (no significant difference for any scaffold type). Mandible scaffolds had an average 4-fold increase in gene expression compared to Proxima Tibias or Vertebral Bodies. For the ATP6v0D gene, the Proximal Tibia scaffold had the highest expression over the other mold types as opposed to the trend of Mandible having the highest gene expressions. Furthermore, this data reveals that the highest relative gene expression comes from genes associated with a more stem-like state (ITGB1, ATP6v0D, TGFbr2) than from a more committed myeloid cell (IL-6, ITGAM).
Figure 2: qPCR analysis was performed on HSC cultured for 10 days in CMP media and grown on 3D scaffolds mimicking the proximal tibia, vertebral body, and mandible. Gene expression for genes associated with the primitive stem cell state (A), Common Myeloid Progenitor state (B), or the monocyte state (C) were quantified using the ddCT method. Cells grown on Mandible scaffolds had higher relative mRNA expression of all genes assessed, except for ATP6v0D, and CSF-1. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Kruskal-Wallis test.
DISCUSSION.
The highest mRNA expressions for all genes tested except ATP6v0D were detected in the mandible scaffolds despite live cells not being able to be detected. It is because of these high mRNA levels that we conclude culturing stem cells in 3D scaffolds mimicking the mandible showed promise towards producing viable cells with a strong, differentiable stem cell-like phenotype. We believe that the inconsistency with the live cell counts and the mRNA for the mandible scaffolds could be due in part to the experimental set up. For the cell counts, we must remove live cells from the 3D culture instead of performing a direct lysis procedure for mRNA collection. It is possible that the cells cultured in the mandible scaffolds could have remained attached to the scaffold and not removed during the accutase procedure due to the mandible scaffold having a larger pore size, pore shape, and curvature [4,9]. Additionally, the automated cell counter used in this analysis had a threshold for the number of cells able to be detected, so it is possible that the number of cells collected from the accutase procedure was too low.
The cells grown in the VB scaffolds (Figure 1) had the highest proliferation over all the other scaffold types, suggesting that stem cells expand best in a 3D culture environment that mimics the vertebral body [9,10]. Since much of the bone marrow in the body is made in the spine, it could be inferred that this is why the cells grow more efficiently here. The data represented by Figure 2 suggest that Lin-HSCs cultured in a mandible-like 3D environment exhibit higher relative gene expression for genes associated with a stem cell phenotype.
For the qPCR, ITBG1, ATP6v0D, TGFbr2, CSF-1, CSF-2, IL-6, and ITGaM were analyzed to identify how specified the stem cells grown got towards the end of the myeloid cell line. Figure 2 shows that all the cells passed the primitive stem cell state and the Common Myeloid Progenitor state, reaching all the way to the monocyte state.
Moving forward with this project, Comprehensive Metabolic Panels (CMPs) will be performed on the cells that were able to be extracted to identify the cell types present. Functional assays will be performed to observe whether the cells can differentiate into macrophages and/or osteoclasts. The results of this study will also be compared to the results of cells grown in 2D culture to see whether there is a significant difference in proliferation.
It is the hope that through these tests, we can grow some fully functioning HSCs to replace the non-functioning HSCs in MDS patients. Since it is the HSCs that become the blood cells in the bone marrow and are the root cause for the total bone marrow failure brought on by MDS, replacing the cells could be the best option to treat the patient. It has the potential to prolong the time before progression to leukemia has a chance to correct some of the bone marrow failure problems.
CONCLUSION.
This research exemplifies a new way of culturing Hematopoietic Stem Cells for future studying of MDS progression. We have shown that HSCs isolated from bone marrow of mice can be cultured in bone mimicking 3D scaffolds and maintain a stem cell-like phenotype. However, the different scaffolds provided different results based on the assay we were observing and should be taken into consideration when designing future experiments for culturing stem cells. VB scaffolds are optimal for growing HSCs as compared to PT and M, while M scaffolds seem to be more optimal for monitoring gene expression.
ACKNOWLEDGEMENTS.
I would like to thank Dr. Julie Rhoades Sterling and the entire Sterling lab for taking me under their wing and mentoring me through this experience. I would also like to thank Dr. Eeds and the School for Science and Math at Vanderbilt for overseeing and giving me this research opportunity.
REFERENCES
- S. Danilin, A. Merkel, R. Johnson, J. Edwards, J. Sterling, Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. OncImmunology, 1(9), 1484-1494 (2012).
- M. Mittelman, H. Oster, M. Hoffman, D. Meumann, The lower risk MDS patient at risk of rapid progression. Elsevier, 34, 1551-1555 (2010).
- D. Buenrosto, S. Park, J. Sterling, Dissecting the Roles of Bone Marrow Stromal Cells on Bone Metastases. BioMed Research International, 1-11 (2014).
- C. Cargo, N. Rowbotham, P. Evans, S. Barrans, D. Bowens, S. Crouch, A. Jack, Targeted sequencing identifies patients with preclinical MDS at high risk of disease progression. Blood Journal, 126(21), 2362-2365 (2019).
- D. Buenrosto, P. Mulcrone, P. Owens, J. Sterling, The Bone Microenvironment: A Fertile Soil for Tumor Growth. Current Osteoporosis Reports, 14(4), 151-158 (2016).
- D. Kerbauy, H. Deeg, Apoptosis and Antiapoptosis mechanisms in the progression of myelodysplastic syndrome. Elsevier, 35, 1739-1746 (2007).
- S. Danilin, A. Merkel, R. Johnson, J. Edwards, J. Sterling, Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. OncImmunology, 1(9), 1484-1494 (2012).
- K. Kwakwa, J. Vanderburgh, S. Guelcher, J. Sterling, Engineering 3D Models of Tumors and Bone to Understand Tumor-Induced Bone Disease and Improve Treatments. Current Osteoporosis Reports, 15(4), 247-254 (2017).
- J. Sterling, J. Edwards, T. Martin, G. Mundy, Advances in the biology of bone metastasis: How the Skeleton affects tumor behavior. Bone, 48(1), 6-15 (2011).
- R. Johnson, M. Nguyen, S. Padalecki, B. Grubbs, A. Merkel, B. Oyajobi, L. Matrisian, G. Mundy, J. Sterling, TGF-β promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. NIH Public Access, 71(3), 822-831 (2011).
Posted by John Lee on Wednesday, December 23, 2020 in May 2020.
Tags: 3D Culture, Myelodysplastic Syndrome, myeloid cells