Investigation of Reaction Thermodynamics Between Carbanion and Carbon Dioxide for Carbon Capture
ABSTRACT
One of the main global contemporary environmental problems is the contribution of CO2 to the atmosphere outpacing Earth’s natural regulation. Cleaning this increasing concentration from the atmosphere is done by carbon capture reactions that utilize carbanion-derived ionic liquids to react with CO2. However, carbon capture reactions possess a high energy cost to initiate, especially on an industrial scale where it would be most applicable. As such, my investigation was into four carbanions to determine which was the most thermodynamically efficient for reacting with CO2. Its scope was focused on analysis of physisorption and chemisorption pathways and determining which carbanion would be the most effective for which pathway. This was determined using 3D chemistry to compare enthalpies, a measure of energy difference between the reactants and products. It was found that molecule C4H4NO3S consistently had the lowest enthalpy value across different products formed from the reaction, indicating the reaction was more exothermic. This means C4H4NO3S would be the most cost-efficient to use in real world carbon capturing. This information is vital in developing cost-efficient methods of carbon capture, so that it is favorable for use on an industrial scale to eliminate excessive CO2 emissions.
INTRODUCTION.
There are a multitude of gases in the atmosphere which through their molecular structure cause the greenhouse effect that maintains Earth’s temperature in a livable range. Carbon dioxide is the primary greenhouse gas, holding more importance than other greenhouse gases in Earth’s atmospheric balancing [1]. This act of atmospheric balancing is an important part of the carbon cycle at large, responsible for the transfers of carbon dioxide between biosphere and atmosphere. The increased CO2 emissions of over 40% for the past 200 years are overpowering Earth’s balancing act to maintain CO2 levels in a healthy range [1]. The concentration of CO2 in the atmosphere pre-industrial revolution was around 277 parts per million (ppm), which after centuries of human mechanization would end up at around 409 ppm in 2019 [2]. The increase has put a hole in the negative feedback loops produced by Earth’s biosphere to maintain atmospheric homeostasis [3]. If left unchecked, global warming from CO2 emissions will contribute to polarizing existing climates, increasing extreme weather events, rising sea levels, and much more from a disruption in this homeostasis [1].
The shift in CO2 concentration in the atmosphere holds several implications for the interconnected elements of Earth’s environment. The Intergovernmental Panel on Climate Change outlined this impact, denoting in a 2018 report that Earth is in danger of surpassing a 1.5 Celsius increase to global mean temperature compared to pre-industrial levels [4]. Changes in temperature to this degree can cause notable complications to global environments. Such changes lead to numerous situations of ice shelf melting, regional droughts, flora and fauna extinction and endangerment, and more as a direct result of temperature shifts [4].
Even whilst posing a grim future for Earth, elements that contribute to this rise in CO2 emissions continue to be used. Human activities make up around 95% of the responsibility behind increased CO2 emissions, which is reflected in the many industrial processes being utilized around the world [5]. Activity commonly seen in countries through modes of road transportation, commercial and residential areas, maritime and aviation transportation, and power generation [5]. Through these humans become responsible for the excess CO2 production as aforementioned, being outlined as a primary contributor for the adverse effects associated with increased CO2 concentrations. However, it is clear that these various fields which produce excess CO2 emissions are necessary components of countries through the goods and services they provide.
Carbon capturing can be utilized to remove CO2 from industrial emissions, and many government incentives and regulatory drives around the world are being used to implement this technology [6]. Carbon capturing can succeed in all matters of power generation and industrial processes, however the only thing standing in its way is the associated cost [7]. As such, this investigation pertains to the thermodynamic analysis of four chosen carbanions to determine which is the most energy efficient for carbon capture. The four chosen were selected from a list of 11 molecules.
The reasons behind why those were chosen are two-fold, one in that molecules 1 and 2 are already extensively studied and promising for carbon capturing; The other reason lies in the subtle differences to their respective acid dissociation constants (pKa) outlining their basicity and as such their effectiveness in a reaction with CO2. The chosen carbanions have a range of basicity, with carbanion 4 being the least basic, followed by 2, then 1 and 3 being the most basic (Figure 1). These four carbanions are C4HN2O2– (1), C4H4NO4S– (2), C4H4NO3S– (4), C3HN2O4– (4) My hypothesis was to test the effect of basicity on reaction enthalpy to determine which investigated molecules would be the most favorable for reacting with carbon dioxide. Carbanions 1 and 2 would effectively serve as the baseline for the comparison as they are molecules already known to be promising for carbon capturing due to their high basicity. If the remaining two were any less or more promising through comparing reaction enthalpies, it would provide important insight.
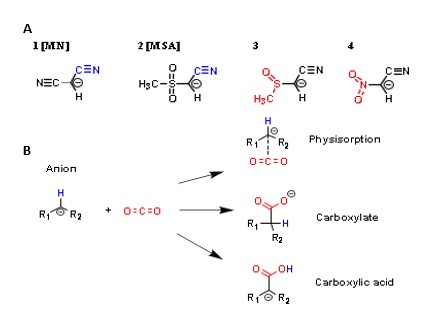
MATERIALS AND METHODS.
3D molecules were constructed primarily using WebMO and Gaussview, both of which are programs for constructing and rendering the computational results that were used for this investigation [8][9].
The four carbanions were studied under two isolated systems, a liquid ionic solvent model and a gas model. These were chosen as the liquid ionic solvent model would be most representative of real-world application of carbon capture, while a gas model is the most standardized for manipulating factors relating to the environment. Within these two models, each physisorption and chemisorption form was investigated to compare enthalpies for all product formations (Figure 1).
To maintain consistency with calculations, as changes in theorems or basis sets would alter results, the same level of theorem and basis set were used in all the molecular calculations. These calculations consisted of both geometric corrections and subsequent thermodynamic calculations to get numerical results. The level of density function theory used was B3LYP, along with this, to ensure accuracy the basis set of 6-311++G(2d,p) was used [8].
The 3D constructed molecules underwent two sets of calculations. The first being geometrical corrections to fix any physical errors with the bond lengths or angles that would impact later results. The second used the corrected molecule to then calculate the thermochemistry.
All calculations resulted in a ΔE value for each molecule found in the output log file, which is representative of the internal energy of a system. The ΔE values were then substituted for their respective molecules in an equation like format and the difference between the reactants ΔE and the product ΔE were calculated (Eq. 1).
\[\Delta E_{product}-\left[\Delta E_{{CO}_2}+\Delta E_{carbanion}\right]=\Delta H_{reaction}\tag{1}\]
Example calculation for Carbanion 1, in Hartree
\[\ \left(-413.1793293\ \right)-\left[\left(-188.652283\ \right)+\ \left(-224.518439\ \right)\right]\ =\ 0.0086073\tag{2}\]
With each new ΔE value found, each one was converted into ΔH, which represents the change in enthalpy as the heat content of the system. ΔE, which was derived directly from the output log was in Hartree units, which was less appropriate to use compared to kJ/mol, a more commonly used value for energy. The final ΔH values were then used in the comparison process to determine the behavior of each carbanion’s reaction to CO2.
The ΔH values allowed the molecules to be quantitatively compared. The comparison will lie in their enthalpies, represented in their ΔH values. Positive values will be indicative of an endothermic reaction, which is indicative of a lower favorability. Negative values will be indicative of an exothermic reaction, indicative of a higher favorability.
This would be done for each investigated carbanion for each of its three product formation pathways. These pathways are relevant due to the real-world application of technology. If a particular molecular is better at physisorption, if a carbon capturer uses the physisorption product pathway then the associated molecule will be more optimized than if it were carboxylic acid or carboxylate. This offers a broader range of applicability for carbanions that denote themselves as the most favorable and increases the effective options for carbon capturing.
RESULTS.
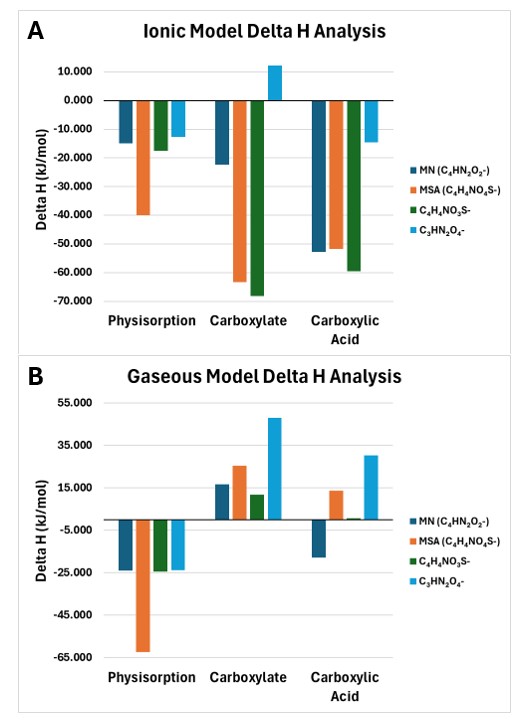
When comparing the ΔH values for each product pathway, a similar trend between the ionic liquid and gaseous model is highlighted. In the gaseous model for physisorption, carbanions 2 and 3 have the highest favorability as denoted by the two lowest ΔH values of –24.35 kJ/mol and –62.20 kJ/mol (Table 1). The same trend is visible in the ionic liquid model, with carbanions 1 and 3 having ΔH values of -39.95 kJ/mol and -17.49 kJ/mol respectively.
Physisorption is the only product formation in the gaseous model wherein carbanion 2 takes a lead in favorability. For carboxylate and carboxylic acid formation though, carbanions 1 and 3 are more favorable. With carboxylate and carboxylic acid in the gaseous model, carbanions 1 and 3 show greater favorability than the other candidates (Table 1).
| Table 1. Enthalpies (ΔH, kJ/mol) of investigated carbanions in the gaseous model | ||||
| MN C4HN2O2– | MSA C4H4NO4S- | C4H4NO3S- | C3HN2O4– | |
| Physisorption | -23.899 | -62.210 | -24.352 | -23.732 |
| Carboxylate | 16.759 | 25.448 | 11.806 | 48.117 |
| Carboxylic Acid | -17.919 | 13.728 | 0.729 | 30.379 |
The ΔH’s 16.75 kJ/mol and -17.91 kJ/mol alongside values 11.80 kJ/mol and 0.72 kJ/mol for their respective molecules are smaller than the ΔH’s of the other two molecules. This also starts to denote the trend of carbanion 4 having the highest ΔH value in all product formations in the gaseous model. It is seen with its value of 48.11 kJ/mol being larger than all the other carboxylate ΔH’s, and its value of 30.37 kJ/mol being larger than all the other carboxylic acid ΔH’s. Both values hold a noticeably large difference to the then second largest ΔH value in both product formation pathways, 22.66 kJ/mol for carboxylate and 16.65 kJ/mol for carboxylic acid.
These gaseous model trends begin to highlight where each molecule stands in the respective product formation pathways. Looking at the ionic liquid model, although used in application for carbon capturing reactions, highlights similar trends identified in the gaseous model. With physisorption, carbanion 2 shows yet again the most favorability (Table 2). Carbanion 3 also presents itself as incredibly favorable in all product formation pathways, similar to its second-place position in the gaseous model.
| Table 2. Enthalpies (ΔH, kJ/mol) of investigated carbanions in the ionic liquid model | ||||
| MN C4HN2O2– | MSA C4H4NO4S- | C4H4NO3S- | C3HN2O4– | |
| Physisorption | -14.891 | -39.950 | -17.490 | -12.740 |
| Carboxylate | -22.284 | -63.406 | -68.262 | 12.265 |
| Carboxylic Acid | -52.748 | -51.788 | -59.530 | -14.507 |
These values though are in fact larger than the values of physisorption of the gaseous model, for example carbanion 2’s ionic liquid solvent model ΔH value being -39.94 kJ/mol which is larger than the -62.20 kJ/mol ΔH of the gaseous model. The value change can be attributed to the environmental characteristics of each model, as an ionic liquid solvent is going to create a different environment for reactions compared to everything being in a gas form. This highlights the focus on identifying general trends relative to their respective models, instead of comparing values between the two models.
Again, carbanion 4 continues to have the highest ΔH values, a trend similar to that of the gaseous model highlighting it as the worst candidate for all product formation pathways (Table 2).
Differentiation in trends between the most favorable molecules in the gaseous and ionic liquid model can start to be seen when comparing the ΔH values for the carboxylate and carboxylic acid product formations. Unlike the gaseous model, carbanion 2 has a higher ΔH value of -63.40 kJ/mol in comparison to 3’s ΔH value of -68.26 kJ/mol for the carboxylate formation (Table 2). Carbanion 4 continues having the highest ΔH value and carbanion 1 is in between carbanion 4 and 2 in favorability (Table 2).
Looking into the carboxylic acid further reveals that carbanion 3 most often has a high favorability. In this case, carbanion 3 has a ΔH value of -59.52 kJ/mol which although slightly smaller than carbanion 1’s ΔH of -52.74 kJ/mol still highlights 3 as the most favorable (Table 2). Once again, Carbanion 4 continues having the highest ΔH value, denoting it as the worst possible candidate.
In comparing these four carbanions in gaseous and ionic liquid models, physisorption, carboxylic acid, and carboxylate are most favorable for carbanion 3. Along with this, carbanions 2 and 1 hold high favorability in a mixture of product formation pathways in both models respectively. However, the high favorability of carbanion 3 outperforms carbanions 1, 2, and 4. Alongside this, it is irrefutable to outline that carbanion 4 has the least favorability in all manner of model and production pathway formation.
In the gaseous model, carbanions 1 and 3 more frequently hold the highest favorability of the four molecules (Figure 2). Along with carbanion 4 holds the least favorability of all product forms. In the ionic liquid model, carbanions 2 and 3 more frequently hold the highest favorability of the four molecules (Figure 2). Again, carbanion 4 being the weakest candidate for carbon capturing.
DISCUSSION.
The investigation’s results mainly provide additional insight into the nature of the four chosen molecules used for carbon capturing. Evidently, as carbanion 3 was more exothermic in its reaction compared to carbanions 1 and 2, the hypothesis of less basic relatives being less favorable to carbanions 1 and 2 can be rejected. However, there was the interesting outcome of carbanion 4 being the least basic yet performing the worse. This implies that there are likely other factors at play aside from just basicity for determining favorability. A good future direction in this case would be to investigate other factors that would impact it, such as environmental conditions like temperature. Regardless, it is evident from the results outlining carbanion 3 as one of the more consistently favorable molecules of the four for most product formation pathways in carbon capturing. This information supports the usage of that particular molecule in carbon capturing to help limit the energy costs. This kind of finding helps provide additional work towards finding the most cost-effective carbanion for it to be justified being used at an industrial scale. It further reinforces the strengths of carbanions 1 and 2 respectively, which provides strong alternatives if a carbon capture process was focused on one specific product formation. The inverse of this is similarly useful to understand, with carbanion 4 being the least favorable in every single category for each model. It reveals which molecules should not be used, eliminating the most inefficient molecules for the development of carbon capture processes.
CONCLUSION.
This investigation focused on identifying the most favorable of four carbanions for carbon capture through the comparison of reaction thermodynamics. The thermodynamics were found through 3D constructed and computationally calculated molecules. Comparison of discovered ΔH values would identify which carbanion was most favorable for which specific product formation pathway. It was identified that carbanion 3 consistently had high favorability in most product formation pathways. This favorability was also seen interchangeably between carbanions 1 and 2, with carbanion 4 being decisively the least favorable in all categories. The theoretical calculations provide this necessary insight into the favorability of these particular molecules. Going forward, this can be utilized in small scale testing to determine if the trends calculated uphold in real world application. Once successfully conducted, look at the cost associated with the small-scale test to determine the correlation of efficiency and cost. Finally, it would provide a means of justification for large-scale implementation to tackle the problem of rising CO2 emissions.
ACKNOWLEDGMENTS.
I would like to first thank the people who even made this all possible through accepting me into a position in their lab, Dr. De-en Jiang. Alongside that, my mentor who worked in conjunction to help me throughout the process of it all Li Bo. Finally, I would like to thank the teachers who made any of this possible to pursue Dr. Joshua Swartz, Dr. Nicholas Means, and Dr. Nathaniel Freymeyer. Everyone listed provided unwavering support that made this all possible.
REFERENCES.
- I. Fung, Read “Climate Change: Evidence and Causes: Update 2020” at NAP.Edu (https://nap.nationalacademies.org/read/25733/chapter/1).
- P. Friedlingstein, et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 12, 3269–3340 (2020).
- J. Rockström, T. Beringer, D. Hole, B. Griscom, M. B. Mascia, C. Folke, F. Creutzig, We need biosphere stewardship that protects carbon sinks and builds resilience. Proc. Natl. Acad. Sci. U.S.A. 118, e2115218118 (2021).
- H. J. Schnellnhuber, W. P. Cramer, Eds., Avoiding Dangerous Climate Change (Cambridge University Press, Cambridge ; New York, 2006).
- M. Mongo, F. Belaïd, B. Ramdani, The effects of environmental innovations on CO2 emissions: Empirical evidence from Europe. Environmental Science & Policy 118, 1–9 (2021).
- S. Budinis, C. Greenfield, M. Fajardy, Carbon Capture, Utilisation and Storage – Energy System, IEA. https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage.
- A. Al‐Mamoori, A. Krishnamurthy, A. A. Rownaghi, F. Rezaei, Carbon Capture and Utilization Update. Energy Tech 5, 834–849 (2017).
- P. William, (2000) WebMO (Version 24.0) [Computer Program]. www.webmo.net
- R. Dennington, (2016) Gaussview (Version 6) [Computer Program]. https://gaussian.com/
Posted by buchanle on Thursday, June 19, 2025 in May 2025.
Tags: Carbanion, Carbon, CO2, Thermodynamics

