Investigating the Roles of TRP Channels in Nociception and Analgesic Applications
ABSTRACT
Transient receptor protein (TRP) channels allow the nervous system to comprehend senses such as sight, smell, taste, and pain, with the sensation of pain being referred to as nociception. TRP channels regulate cation influxes which control neural membrane potential and activation from potentially dangerous stimuli which lead to influxes causing signal propagation across neurons until the nervous system recognizes pain and elicits a pain response. After analyzing the literature regarding dysfunction of nociceptive TRP channels and the various pain-related conditions damages are associated with, links have been found between nociceptive TRP channel mutations and impairments in sensing temperature and pain. Attempts have been made to exploit TRP channel function to pain-relievers, of which capsaicin desensitization has proven to be most effective.
INTRODUCTION.
Transient receptor protein (TRP) channels are transmembrane proteins that primarily mediate the flow of Ca2+, Na+, and Mg2+ cations across the cell membrane. TRP channels allow for the sensation of physical and chemical external stimuli, pH, temperature, and activation ligands [1]. TRP channels act as signal transduction initiators in response to external stimuli, which allows for the sensations of sight, smell, taste, and pain.
Physiologically, TRP channel function varies widely, from mediating ions to regulating neuron membrane potentials [1-2]. Therefore, dysfunction of TRP channels results in a wide array of disorders [1-2]. However, the scope of this literature review is to specifically assess the structure, function, and result of dysfunction of nociceptive TRP channels.
TRP Channel Structure. The TRP family consists of six sub-families totaling 28 different known proteins [3]. The six families are: canonical (TRPC), vanilloid (TRPV), ankyrin (TRPA), melastatins (TRPM), mucopilins (TRPM1), and polycystin (TRPP) families [4]. These TRP families play significant roles in a variety of physiological processes, with TRPV, TRPA, and TRPM channels interplaying significantly in nociception [4].
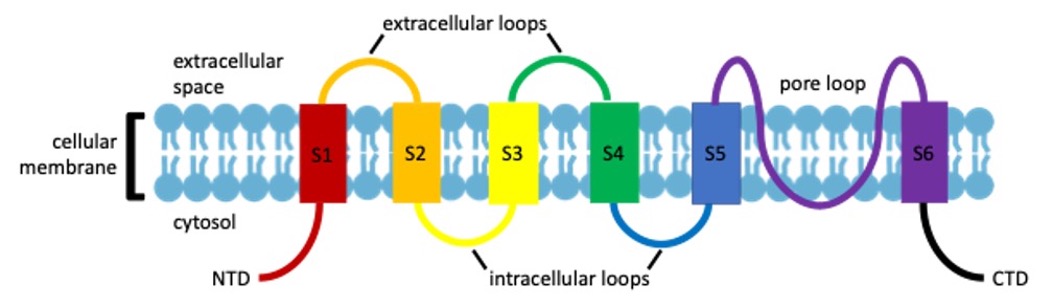
Based on structural data, TRP channels are characterized by their six transmembrane domains. The first four transmembrane domains function as the voltage sensor and agonist binding site, where a hydrophilic loop between the fifth and sixth transmembrane domains form the ion conducting pore (Fig. 1) [5]. The position of amino acid residues within this loop varies between channels, determining its selectivity for different cations [6].
Figure 1. Basic Protein Domain Monomeric Structure of TRP Channels. S1-S6 are the membrane spanning domains. NTD=N-Terminal Domain; CTD=C-Terminal Domain.
Nociceptive TRP Channel Activation and Cation Regulation. Amino acid sequence and length at the N- and C-termini vary across different TRP channels, creating the diversity within the TRP family [7]. Of the six subfamilies, only TRPV, TRPA, and TRPM channels are considered “nociceptive TRP channels,” activated by painful or noxious stimuli. Within these groups, TRPV1, TRPA1, and TRPM8 have been identified to play more significant roles in nociception [6].
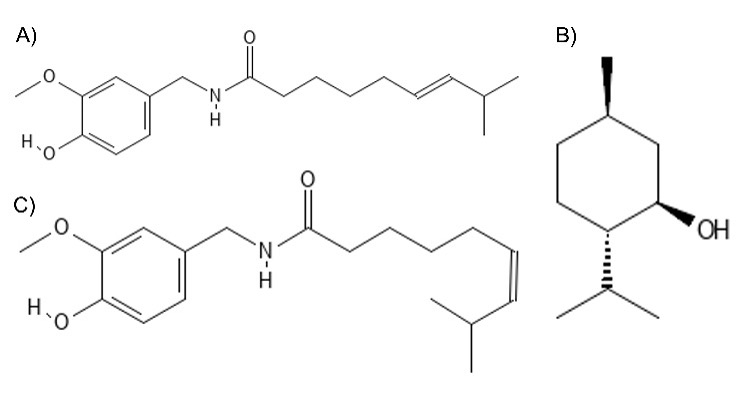
TRPV1 channels are expressed in nociceptive neurons throughout the body and therefore play a highly significant role in pain sensation, activated by a variety of stimuli including noxious heat, protons, and chemical ligands [7]. Furthermore, TRPV channels are unique in how they can be activated by the vanilloid ligand capsaicin (Fig. 2A) commonly found in chili peppers [7]. Upon TRPV1 capsaicin activation, there is an excitation period followed by a refractory period where the cells stop responding to other stimuli [7]. TRPV1 channels are also terrific heat sensors, activated by temperatures over 42°C, [7] TRPA1 and TRPM8 activate in response to a decrease in temperature (<20°C), with activation in the dorsal root and trigeminal ganglion sensory neurons resulting in the cold sensation [8]. TRPM8 is also chemically activated by cooling compounds such as menthol (Fig. 2B), found naturally in mint plants [1,8]. Other channels that also play a role in nociception include the high temperature activated TRPM3, TRPV2 and TRPV3 [9]. It is hypothesized that molecular damage of TRP channels leads to the dysregulation of sensory induction, lessening the ability to sense pain, perhaps by the desensitization to both thermal and chemical external stimuli.
Figure 2. Molecular Structures of Compounds that Activate TRP Channels. A) Capsaicin; B) Menthol; C) Zucapsaicin.
MATERIALS AND METHODS.
Literature Curation. This literature review was conducted by surveying the current relevant literature on TRP channels published on credible databases such as the National Center for Biotechnology Information (NCBI), Google Scholar, and bioRXIV. Keywords for the literature curation included TRP channels, nociception, pain management, membrane cation flow, and other related keywords. Articles referenced date as far back as 2008 and as current as 2022.
RESULTS.
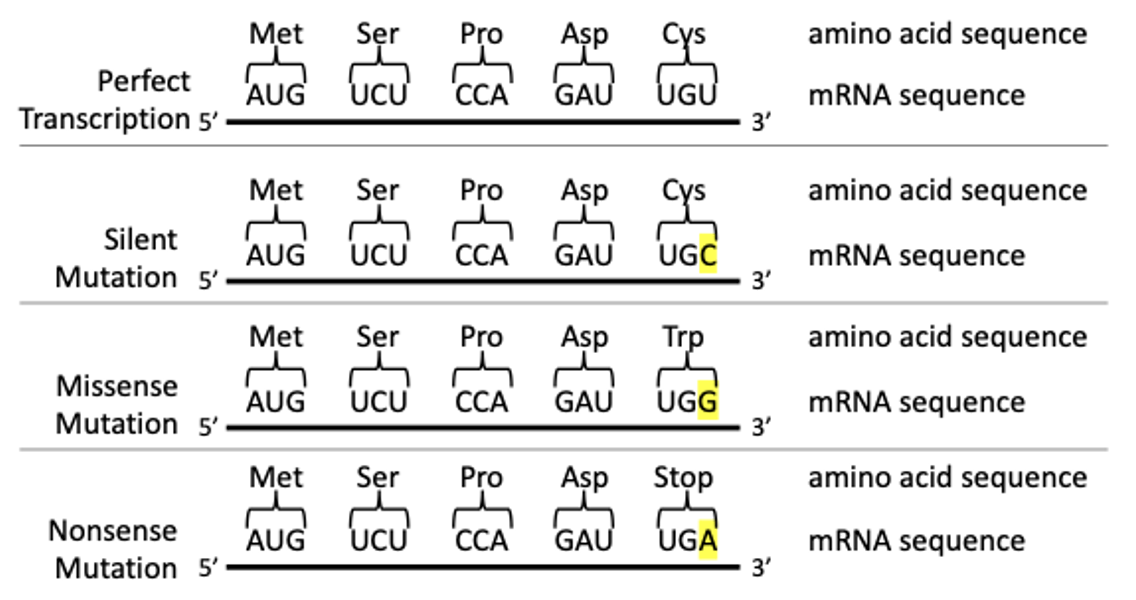
Classifying Mutations. After reviewing numerous articles on TRP channel mutations and the impacts of dysregulation, point mutations, the replacement of a single nucleotide base, is the primary origin of TRP channel variants. Point mutations can be grouped into three types: silent, missense, or nonsense mutations (Fig. 3) [18, 19]. Mutations to a nociceptive TRP channel can either leave its structure unchanged or altered, the latter potentially leading to dysfunction and modulations in how pain is perceived.
Figure 3. Mutation Types and their Effect on Protein Sequence/Structure. Silent mutations result in no change to the amino acid sequence. Missense mutations result in the substitution of an amino acid and could potentially result in the change of the protein secondary structure. Nonsense mutations result in a premature stop codon, typically yielding a non-functional protein.
Following a missense or nonsense mutation, the protein will either lose their original function, resulting in a Loss of Function (LOF) mutation, or gain a new function, resulting in a Gain of Function (GOF) mutation [20]. In TRP channels, GOF mutations predominantly reside over LOF mutations [18]. It is hypothesized that the domination of GOF mutations is due to LOF mutations having serious implications in embryonic development due to their lethal-null phenotypes.
TRP Mutation Phenotypes. Several studies investigating the impacts of TRP channel point mutations on sensation have found the following mutations to impact nociceptive abilities. With the exception of TRPA1 E179K, the domains of the investigated mutants are unknown.
TRPV1. Duo et al. (2018) determined the impacts of the G564S GOF mutation in TRPV1 channels in mice and demonstrated that G564S mice displayed premature TRPV1 channel activation before capsaicin stimulation occurred [5]. After repeated doses of capsaicin, wild-type mice showed a decreased reaction to subsequent doses, while those with the G564S mutation did not, implicating that the mutation overall decreases TRPV1 function and sensitivity to noxious heat [5].
Abeele et al. (2019) demonstrated that the Y524S mutation in murine TRPV1 channels resulted in abnormally high hypermetabolic response when exposed to higher temperatures, with increased efficacy of TRPV1 agonists countering these responses [11]. This demonstrates that TRPV1 dysfunction not only plays a role in malignant hyperthermia, but also that agonists could be a putative treatment for hyperthermia [11].
TRPA1. TRPA1 N855S has been identified as a key player in familial episodic pain syndrome, a genetic disorder characterized by episodes of recurring neuropathic pain [12]. The mutation drastically increases neuronal cation influx upon activation [12]. However, a subsequent investigation by Kremeyer et al.(2010) demonstrated that both wild-type and N855S subjects showed no difference in stimulus detection thresholds [12].
TRPA1 E179K, located in the protein’s ANK 3 domain, is often found in patients experiencing paradoxical heat sensation (PHS), where patients feel burning heat when exposed to noxious cold stimuli [13]. It has been hypothesized that the mutation alters the channel’s secondary structure as the hydrophilic glutamate in position 179 is substituted by the hydrophobic lysine, altering how the amino acids interact with one another [14].
Other TRP channels. It has been reported that an unspecified TRPV3 mutation correlates to the onset of Olmsted syndrome, with symptoms including keratoderma (abnormal thickening of the epidermis), alopecia, and severe itching [15]. Lin et al. (2012), propose that this mutation increases TRPV3 activation and henceforth Ca2+ levels in keratinocytes [15].
TRPM8 R30Q, has been found in patients with trigeminal neuralgia, a chronic condition linked to severe pain in the face [16-17]. However, Gualdani et al.(2021) did not observe direct causation between R30Q and trigeminal neuralgia, but found that the variant increased TRPM8 activation in response to menthol and increased intracellular Ca2+ [17].
TARGETING TRP CHANNELS FOR PAIN RELIEF.
The corroboration by many studies indicates that TRP channel damage modulates nociception; therefore, targeting nociceptive TRP channels is a putative strategy for pain relief. This putative strategy would be preferred as other analgesics on the market have their limitations; with non-steroidal anti-inflammatory drugs (NSAIDs) having limited use in high degrees of pain and opioid usage being effective but associated with high risk of abuse [21]. Antagonists of TRPV1 and other nociceptive TRP channels have been widely explored for therapeutic potential, following the hypothesis that hindering nociceptive TRP channels would modulate nociception.
Capsaicin Desensitization. Using TRPV1 agonists as an analgesic exploits the refractory period followed by activation, desensitizing the channel. Capsaicin has been explored in depth for pain desensitization. Trials examining capsaicin exposure found desensitization to be reversible, suggesting the treatment is not necessarily 100% reliant on TRPV1 [22]. Nevertheless, products containing low concentrations of capsaicin have been available over the counter since the 1980s as self-administered remedies [23].
Qutenza (NGX-4010), an 8% capsaicin adhesive patch is one medication that has successfully undergone clinical trials [24]. In November 2009, Qutenza was FDA approved and since then patches have been available both by prescription and over the counter [25]. Zucapsaicin (Fig 2C), the cis-isomer of capsaicin, modulates TRPV1, reducing pain and treating chronic nerve pain conditions [26]. While Zucata, a cream containing Zucapsaicin (Fig 2C), was approved by Health Canada, Zucapsaicin is currently not FDA-approved [26].
TRP Antagonists. Previous studies on rodents have shown that administering small-molecule TRPV1 antagonists can reduce inflammation-associated hyperalgesia [6]. These results, along with other pre-clinical research, encouraged testing nociceptive TRP antagonists for pain relief [6]. TRPV1 antagonist AZD1386 made it to phase II in clinical trials, which were later terminated.
Because TRPV1 plays a key role in maintaining body temperature, regular doses of antagonists impairing TRPV1 function were found to lead to hyperthermia, implying that antagonists would not be sustainable treatment [27]. Other TRP channels antagonists which have completed phase II clinical trials include GRC-17536 targeting TRPA1 and SAR292833 targeting TRPV3, both for neuropathic pain, with unpublished results [6].
CONCLUSION.
TRP channel variants result in the modulation of TRP channel biochemical processes (basal nociception). However, TRP channel defects that result in the structural alteration of the channel result in widely ranging functional defects perturbing normal nociception and are associated with various diseases. Recorded causes of channel damages altering their structures and functions are limited, potentially indicating that not only has pain sensation been evolutionarily important to survival, but also that catastrophic TRP channel damages likely result in an embryonic lethality, emphasizing their importance to survival. This is further corroborated by all reviewed cases of nociceptive TRP channel damage stemming from GOF, not LOF mutations associated with conditions concerning pain and temperature sensation or itching.
While protein structure to protein function has been mapped on TRP channels, there are still regions of unknown function that require further exploration to ascertain a more global perspective regarding TRP channel structure and function. A prime example of this is the fact that the majority of the mutations listed in this study are found in regions of unknown domain function. As research progresses, viewing how altered TRP channels impact pain sensation can hopefully be applied in future clinical trials targeting TRP channels and their antagonists for development in analgesic production.
ACKNOWLEDGEMENTS.
I acknowledge Lumiere Education for facilitating and funding this research. I would also like to thank my family for their consistent support of my academic interests.
REFERENCES.
- Zheng. Molecular mechanism of TRP channels. Comp. Phys. 3,1 (2013).
- Alaimo, J. Rubert. The Pivotal Role of TRP Channels in Homeostasis and Diseases throughout the Gastrointestinal Tract. Intl. Jour. Mol. Sci. 20(21), 5277 (2019).
- P. Koivisto. M.G. Belvisi, R. Gaudet, A. Szallasi. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Disc., 21(1), 41–59 (2022).
- Jardín., J. J. López, R. Diez., J. Sánchez-Collado, C. Cantonero, L. Albarrán, G. E. Woodard., P. C. Redondo, Salido, G. M., Smani, T., & Rosado, J. A. TRPs in Pain Sensation. Front. Phys. 8, 392 (2017).
- Duo, L., Hu, L., Tian, N., Cheng, G., Wang, H., Lin, Z., Wang, Y., & Yang, Y. TRPV1 gain-of-function mutation impairs pain and itch sensations in mice. Pain.14, 1744806918762031 (2018).
- D. Mickle, A. J. Shepherd, D. P. Mohapatra, Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals, (Basel, Switzerland), 9(4), 72 (2016).
- A. Hellmich, R. Gaudet, Structural biology of TRP channels. Handbook of experimental pharmacology, 223, 963–990 (2014).
- Xu, Y. Han, X. Chen, A. Aierken, H. Wen, W. Zheng, H. Wang, X. Lu, Z. Zhao, C. Ma, P. Liang, W. Yang, S. Yang, F. Yang. Molecular mechanisms underlying menthol binding and activation of TRPM8 ion channel. Nature communications, 11(1), 3790 (2020).
- Vriens, G. Owsianik, T. Hofmann, S. E. Philipp, J. Stab, X. Chen, M. Benoit, F. Xue, A. Janssens, S. Kerselaers, J. Oberwinkler, R. Vennekens, T. Gudermann, B. Nilius, T. Voets,. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron, 70(3), 482–494 (2011).
- M. McEntire, D.R. Kirkpatrick, N.P. Dueck, M.J. Kerfeld, T.A. Smith, T.J. Nelson, M.D. Reisbig, D.K. Agrawal. Pain transduction: a pharmacologic perspective. Expert review of clinical pharmacology, 9(8), 1069–1080 (2016).
- Vanden Abeele, S, Lotteau, S. Ducreux., C. Dubois, N. Monnier, A. Hanna, D. Gkika, C. Romestaing, L. Noyer, M. Flourakis, N. Tessier, R. Al-Mawla, C. Chouabe, E. Lefai, J. Lunardi, S. Hamilton, J. Fauré, F. Van Coppenolle, N. Prevarskaya TRPV1 variants impair intracellular Ca2+ signaling and may confer susceptibility to malignant hyperthermia. Genetics in medicine: official journal of the American College of Medical Genetics, 21(2), 441–450 (2019).
- Kremeyer, F. Lopera, J.J. Cox, A. Momin, F. Rugiero, S. Marsh, C.G. Woods, N.G. Jones, K.J. Paterson, F.R. Fricker, A. Villegas, N. Acosta, N,G. Pineda-Trujillo, J.D. Ramírez, J. Zea, M.W. Burley, G. Bedoya, D.L. Bennett, J.N. Wood A. Ruiz-Linares. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron, 66(5), 671–680 (2010).
- May, J. Baastrup, M.R. Nientit., A. Binder, M. Schünke, R. Baron, I. Cascorbi. Differential expression and functionality of TRPA1 protein genetic variants in conditions of thermal stimulation. The Journal of biological chemistry, 287(32), 27087–27094 (2012).
- Binder, D. May, R. Baron, C. Maier, T.R. Tölle, R.D. Treede, A. Berthele, F. Faltraco, H. Flor, J. Gierthmühlen, S. Haenisch, V. Huge, W. Magerl, C. Maihöfner, H. Richter, R. Rolke, A. Scherens, N. Uçeyler, M. Ufer, G. Wasner, I. Cascorbi. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PloS one, 6(3), e17387 (2011).
- Lin, Q. Chen, M. Lee, X. Cao, J. Zhang, D. Ma, L. Chen, X. Hu, H. Wang, X. Wang, P. Zhang, X. Liu, L. Guan, Y. Tang, H. Yang, P. Tu, D. Bu, X. Zhu, K. Wang, R. Li, R., … Y. Yang, Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. American journal of human genetics, 90(3), 558–564 (2012).
- Rivera, C. Moreno, B. Lavanderos, J.Y. Hwang, J., Fernández-Trillo, K.S. Park, P. Orio, F. Viana, R. Madrid, M. Pertusa, Constitutive Phosphorylation as a Key Regulator of TRPM8 Channel Function. The Journal of neuroscience: the official journal of the Society for Neuroscience, 41(41), 8475–8493 (2021).
- R, Gualdani, Y. J. Yuan, P.R. Effraim, G. Di Stefano, A. Truini, G. Cruccu, S.D Dib-Hajj, P. Gailly, S.G. Waxman Neurol Genet, 7 (1) e550 (2021).
- Yuan, W. Wang, H. Li, Y. Yu, J. Tao, S. Huang, Z. Zeng. Nonsense and missense mutation of mitochondrial ND6 gene promotes cell migration and invasion in human lung adenocarcinoma. BMC cancer, 15, 346 (2015).
- R. Patel, S. Gautam, D. Chatterji. Unraveling the Role of Silent Mutation in the ω-Subunit of Escherichia coli RNA Polymerase: Structure Transition Inhibits Transcription. ACS omega, 4(18), 17714–17725 (2019).
- Li, Y. Zhang, X. Li, S. Yi, J. Xu. Gain-of-Function Mutations: An Emerging Advantage for Cancer Biology. Trends in biochemical sciences, 44(8), 659–674 (2019).
- D. Brederson, P.R. Kym, A. Szallasi. Targeting TRP channels for pain relief. European journal of pharmacology, 716(1-3), 61–76. (2013).
- Arora, J.N. Campbell, M.K. Chung. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacology & therapeutics, 220, 107743 (2021).
- Y. Wong, N.R. Gavva. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain research reviews, 60(1), 267–277(2009).
- F. Peppin, K. Majors, L.R. Webster, D.M. Simpson, J.K. Tobias, G.F. Vanhove. Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. Journal of pain research. (2011).
- Stewart, J. (n.d.). Qutenza (capsaicin) FDA approval history. Drugs.com
- Sałat, A. Jakubowska, K. Kulig. Zucapsaicin for the treatment of neuropathic pain. Expert opinion on investigational drugs, 23(10), 1433–1440 (2014).
Posted by John Lee on Tuesday, May 30, 2023 in May 2023.
Tags: Analgesics, Nociception, TRP Channels, TRP Mutations