Evaluating the Efficacy of Combined 5-Fluorouracil and Paclitaxel Chemotherapy Relative to Monotherapy in Treating Gastric Cancer
ABSTRACT
5-Fluorouracil (5-FU) is a chemotherapeutic agent commonly used to treat multiple cancer types, such as breast, colon, rectal, pancreatic, and gastric cancer. The antitumor activity of 5-FU comes from its antimetabolic ability to inhibit DNA synthesis. However, its effectiveness as an anticancer therapy is often limited by its poor toxicity profile and the rapid development of drug resistance in tumor cells, ultimately reducing its overall efficacy as a monotherapy. Therefore, combining 5-FU with other antineoplastic agents to reduce its administrated dose may not only potentially lower the incidence of toxicity but also enhance the antitumor effect. In this study, we treated gastric cancer MKN-45 cells with 5-FU monotherapy, Paclitaxel (PTX) monotherapy, and a combination therapy of 5-FU plus PTX at different doses. Greater rates of cytotoxicity in MKN-45 cells treated with combination therapy compared with either monotherapy group were found, suggesting that administering 5-FU in combination with other anticancer therapy potentiates the antitumor effect of monotherapy, and might be a mean to decrease the incidence of 5-FU–dependent toxicities by reducing its administered dose.
INTRODUCTION.
5-Fluorouracil (5-FU) is a chemotherapy drug commonly used for treatments of head, neck, breast, prostate, pancreatic, liver, genitourinary and gastrointestinal tract carcinomas [1]. An antimetabolite, 5-FU interferes with cancer growth by acting as a substitute for the normal building blocks of RNA and DNA [2]. By acting as a substitute for uracil, 5-FU can disrupt nucleotide synthesis. Additionally, 5-fluororuacil inhibits thymidylate synthase (TS), a critical enzyme in the production of nucleotides required for DNA replication [3]. Specifically, TS is responsible for the conversion from 2′-deoxyuridine-5′-monophosphate (dUMP) to 2′-deoxythymidine-5′-monophosphate (dTMP), also known as thymidylate, a nucleotide required for DNA synthesis [3]. The inhibition occurs through 5-fluoro-dUMP (FdUMP), the active metabolite of 5-FU, which binds to TS and CH2-THF [3]. This process forms a stable complex that interrupts the functions of TS, causing scarcity of dTMP and ultimately blocking DNA synthesis. [3]. By disrupting DNA synthesis, 5-FU effectively prevents cell proliferation in rapidly dividing cancer cells, causing cell death.
Although 5-FU is the most commonly administered first-line treatment for many cancer types, treatment with 5-FU often results in myelosuppression (decreased bone marrow production), dermatitis (skin irritation), cardiac toxicity, and causes toxicity in the gastrointestinal tract in 40–80% of patients [4]. This poor tolerability profile limits the drug’s applicability for many patients. Additionally, the efficacy of 5-FU is hindered by the various mechanisms with which tumor cells can develop resistance to the drug [5]. Furthermore, mono-therapeutic, or single-drug, clinical utilization of systemic fluorouracil therapy has shown low efficacy due to various mechanisms of drug resistance, with only a 10% to 15% overall response rate in patients with advanced colorectal cancer [5]. However, research has shown that when administered in combination with other antineoplastic agents—drugs that inhibit or prevent tumor growth—the efficacy of fluorouracil-based treatments can be augmented, with response rates rising to between 40% and 50% [5].
One such antineoplastic agent, paclitaxel (PTX), is often administered in patients with gastric cancer as second-line chemotherapy following first-line treatment with 5-FU because of its proven efficacy against cancer and favorable tolerability among patients who have discontinued other therapies due to toxicity [6]. PTX acts via a molecular pathway distinct from that of 5-FU, namely, as an antimitotic and antimicrotubular agent [7]. PTX binds to β-tubulin subunits within microtubules, which are structural components of the cell that help maintain its shape and play a crucial role in cell division. This process promotes the assembly and stabilization of microtubules [7]. The increased stability of microtubules hinders their ability to undergo the normal process of depolymerization (the disassembly of microtubules), thus disrupting the formation and functionality of the mitotic spindle and consequently inhibiting the segregation of chromosomes during mitosis [7]. Improper spindle formation triggers and activates the spindle assembly checkpoint (SAC), a cellular mechanism that detects anomalies in chromosomal alignment and attachment to the spindle, halting the cell cycle at the G2/M phase and ultimately leading to programmed cell death (apoptosis) [7].
Because treatment with 5-FU carries with it a high risk of toxicity, lowering the administered dose of 5-FU by combining it with other therapeutic agents is desirable. [8] It may be possible that, by administering each agent at a reduced concentration, the risk of toxicity resulting from each individual therapy can be reduced. Additionally, by selecting a combination agent which acts via a distinct molecular pathway from 5-FU, it is possible that undesirable drug toxicity and resistance outcomes of monotherapy agent can be further mitigated. [8] Therefore, it is worthwhile to investigate whether administering 5-FU plus PTX as combination therapy result in increased rates of cell death in cancer cells, even when the dose concentrations of each individual agent are reduced compared to their respective mono-therapeutic doses.
The relative efficacy of 5-FU plus PTX, 5-FU monotherapy, and PTX monotherapy can be evaluated by comparing rates of cell death, as measured by MTT cell viability assay. By treating MKN-45 cells, a gastric cancer cell line, with progressively greater concentrations of 5-FU and PTX both individually and in combination, we observed reduced cell viability in 5-FU plus PTX groups compared with both 5-FU and PTX monotherapy groups, suggesting that a combination therapy comprising both agents has the potential for greater efficacy in gastric cancer patients.
MATERIALS AND METHODS.
Cell Culture.
Human gastric cancer MKN-45 cell line was obtained from Bioresource Collection and Research Center (BCRC, Taiwan). The cells were cultured in RPMI 1640 medium (Invitrogen, 1187093), supplemented with 10% fetal bovine serum (FBS [Sigma-Aldrich, F7524]), 2 mM L-glutamine, 1% sodium pyruvate, and 2% antibiotic at 37°C in a humidified atmosphere of 5% CO2, resuspended by trypsin with 0.25% Ethylenediaminetetraacetic acid (EDTA) and collected.
Cell Viability Assay.
MKN-45 cells were seeded into sterile 24-well plates with 2*104 cells/well. After treatment, 3- (4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H- tetrazolium bromide (MTT [Siga-Aldrich, 852065]) solution was added to each well at a final concentration of 0.5 mg/ml per well and the plates were incubated at 37°C for 2.5 hours. Following incubation, the supernatant medium in the 24 wells was removed, and dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan. The absorbance was read at 595 nm using a spectrophotometric microplate reader (TECAN, Switzerland).
Statistical Analysis.
Each experiment was performed at least three times independently under identical conditions, and data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were conducted using GraphPad Prism 9.0 (GraphPad Software Inc., La Jolla, CA, USA); one-way Analysis of Variance (ANOVA) was carried out for multiple comparisons. Fisher’s Least Significant Difference (LSD) test was used to assess substantial correlations in cytotoxicity effects. Additionally, one-way ANOVA was used to determine the correlation between 5-FU and PTX’s cytotoxic effects on MKN-45 cell lines. Statistical probability (p) is expressed as *p<0.05, **p<0.01, and ***p<0.001 (p-value of less than 0.05 was considered statistically significant).
RESULTS.
We cultured MKN-45 cells and separated them into three groups (5-FU monotherapy, PTX monotherapy, and their combination). Drug treatment concentrations for each group were dosage-dependently designed. After treatments, cells of each group were separated into individual 24-well plates; each plate contained 2 wells of cells that did not receive treatment (control groups). 5-FU monotherapy was administered at concentrations of 2, 5, 10, and 20 μM; PTX monotherapy was administered at concentrations of 1, 2, and 5 ng/ml. Combination treatment was administered at 6 different doses: 5-FU was administered at concentrations of 1,2, and 5 μM ; each of these dosing groups was separated into two dosing groups for PTX (1ng/mL and 2 ng/mL). All three treatment groups were subjected to treatment over a 72-hour period, with the MTT cell viability assay being collected for the 48-hour and 72-hour marks. Due to a laboratory error, 72-hour data was not collected for the combination treatment group.
Data from all three treatment groups displayed statistically significant reductions in cell viability (Fig. 1, 2, 3). We observed statistically significant reductions in cell viability at both the 48- and 72-hr marks for all dosing groups when 5-FU was administered alone (Fig. 1); when PTX was administered alone, statistically significant reductions were only observed at both the 48- and 72- hour mark for the 2 ng/mL and 5 ng/mL dosing groups. For the 1 ng/mL dosing group, statistically significant reduction was only observed at the 72-hour mark.
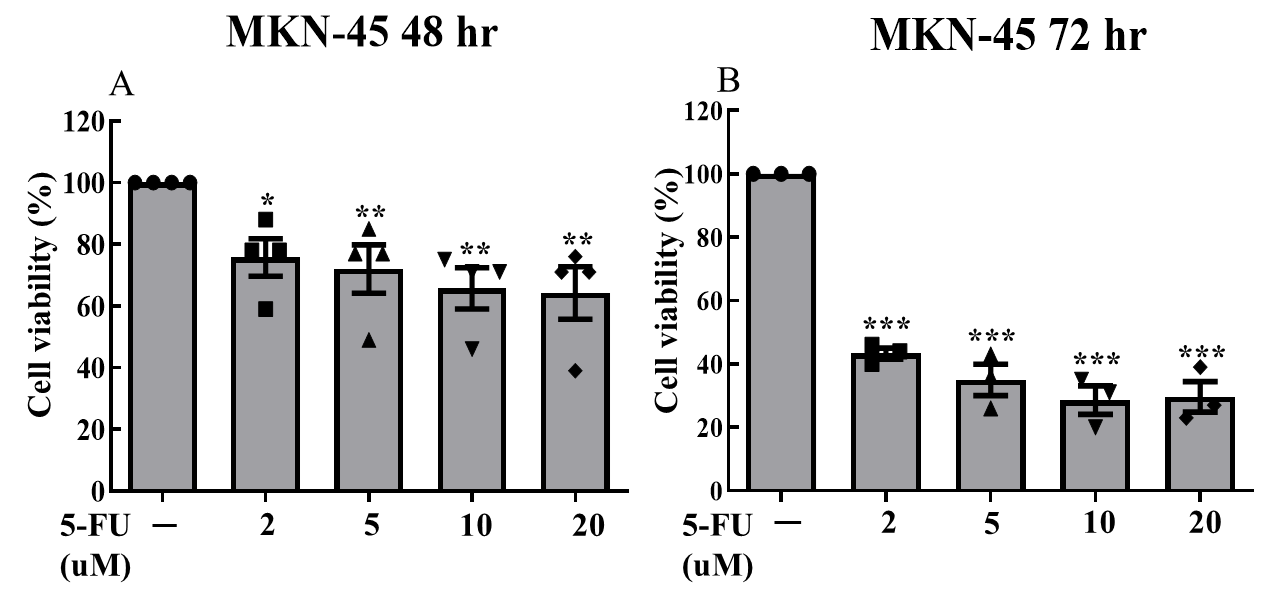
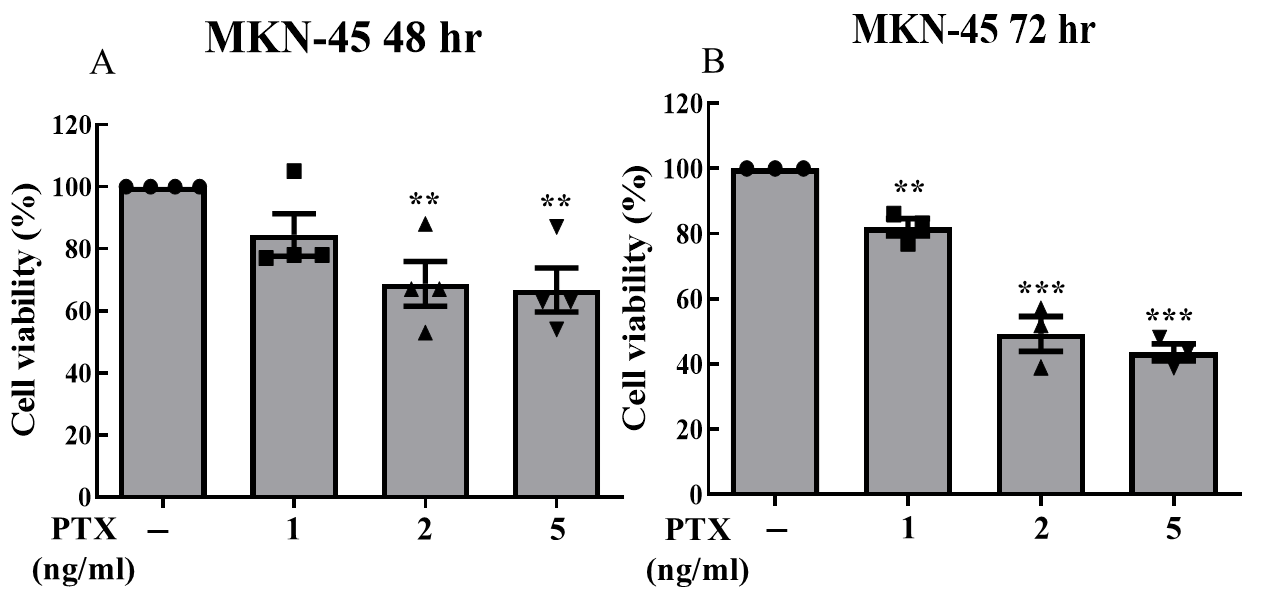
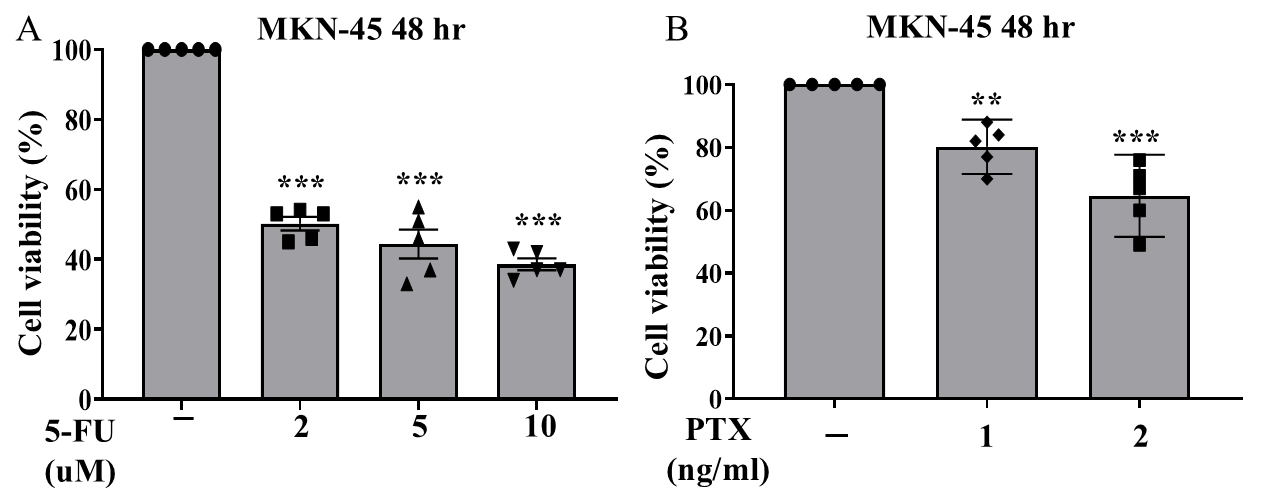
Of the three treatments administered, the combination of 5-FU plus PTX showed the lowest rates of cell viability, suggesting greater efficacy in treating gastric cancer cells compared with either individual agent as monotherapy. However, the addition of PTX did not always correlate with greater observed cytotoxicity; in fact, cells treated with 10 uM 5-FU + 2 ng/mL PTX actually displayed greater rates of viability than those treated with 10 uM 5-FU and 1 ng/mL PTX, suggesting that the reductions in cell viability observed in the combination therapy treatment groups may have resulted more from the presence of 5-FU than that of PTX.
DISCUSSION.
Despite its ubiquity as a first-line therapeutic agent for a broad range of cancer types, 5-FU treatment poses several logistical challenges. High rates of multiple toxicities resulting from the treatment pose an obstacle to continued treatment in many cancer patients. Furthermore, rapid development of drug resistance in tumor cells can make long-term treatment with 5-FU ineffective, even with patients who tolerate treatment. Assessing the relative efficacy of combination therapies including 5-FU and other therapeutic agents, compared with monotherapy alone, can therefore suggest alternative treatment pathways with the potential to reduce incidence of toxicity and improve response times prior to relapse.
In this study, we used gastric cancer MKN-45 cells to evaluate the relative efficacy of 5-FU plus PTX combination therapy compared to each individual agent as monotherapy. Using MTT cell viability assay, we observed that the combination therapy resulted in greater rates of cell death than either of the individual therapies administered alone (Fig. 3, 4). While cell viability rates were not greatly reduced when comparing the combination treatment to 5-FU monotherapy, the data suggests that combination therapy can provide a pathway to reducing administered doses of 5-FU, thereby potentially reducing incidence of toxicity and improving treatment effect for patients.
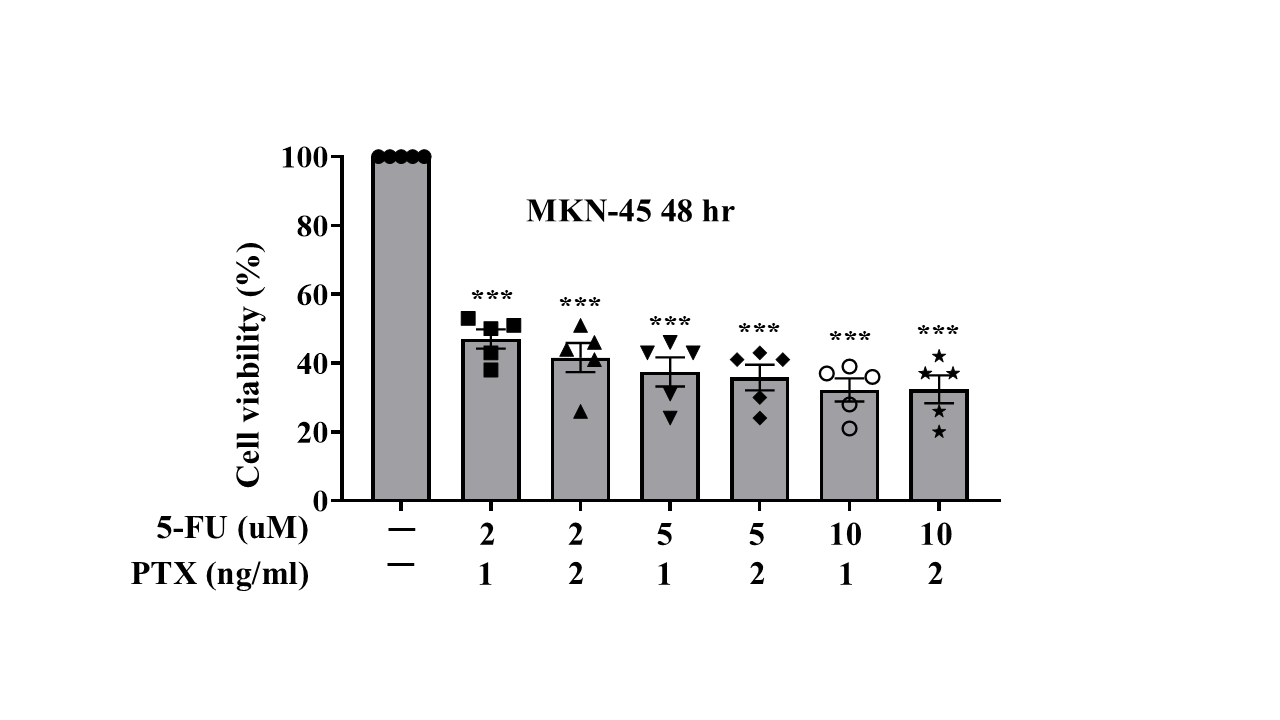
It is possible that in the combination treatment group, observed reduction in cell viability is largely due to the presence of 5-FU, rather than PTX. Indeed, when comparing the cell viability rates between cells treated with 10um of 5-FU + 1 ng/mL of PTX and those treated with 10um of 5-FU + 2ng/mL of PTX, cell viability improved in the latter group, suggesting that the presence of PTX may have been negligible in contributing to cytotoxicity in the combination group (Fig. 3, 4). That said, the reduction in cell viability observed in combination therapy groups compared with that of monotherapy suggests that other combination agents may be promising alternatives. Further analyses conducted using a variety of combination agents with 5-FU will surely offer more insight. Moreover, re-challenging cells with either combination therapy or monotherapy and comparing their subsequent rates of cell viability can offer further clues as to what treatment pathways exist that may reduce incidence of formation of drug resistance in tumor cells.
ACKNOWLEDGMENTS.
I would like to express my gratitude to my mentors for supporting and guiding me throughout this entire research project. This research opportunity was ultimately made possible by National Cheng Kung University Hospital, which graciously provided me with the opportunity and necessary resources to carry out this research.
REFERENCES
1 G.V. Koukourakis, V. Kouloulias, M.J. Koukourakis, G.A. Zacharias, H. Zabatis, J. Kouvaris, Efficacy of the Oral Fluorouracil Pro-drug Capecitabine in Cancer Treatment: a Review. Molecules. 13, 1897–1922 (2008).
2 D.B. Longley, D.P. Harkin, P.G. Johnston. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 3, 330–338 (2003).
3 G.J Peters, H.H.J Backus, S. Freemantle, B. van Triest, G. Codacci-Pisanelli, C.L. van der Wilt, K. Smid, J. Lunec, A.H. Calvert, S. Marsh, H.L. McLeod, E. Bloemena, S. Meijer, G. Jansen, C.J. van Groeningen, H.M. Pinedo, Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease. 1587, 194–205 (2002).
4 D. Rodrigues, T. de Souza, L. Coyle, M. Di Piazza, B. Herpers, S. Ferreira, M. Zhang, J. Vappiani, D.C. Sévin, A. Gabor, A. Lynch, S.W. Chung, J. Saez-Rodriguez, D.G.J. Jennen, J.C.S. Kleinjans, T.M. de Kok. New insights into the mechanisms underlying 5-fluorouracil-induced intestinal toxicity based on transcriptomic and metabolomic responses in human intestinal organoids. Arch Toxicol. 95, 2691–2718 (2001).
5 N. Zhang, Y. Yin, S.J. Xu, W.S. Chen. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules. 13, 1551–1569 (2008).
6 A.K. Madeeh, H.K. Farouk, M.M. Belal, S. Ramadan, B.E. Al-Masri, M. Samier, S.A. Gadallah, N. Khapoli, A.J. Nashwan, Y.H. AbdelQadir. Efficacy and safety of nab-paclitaxel chemotherapy for patients with gastric carcinoma: a systematic review and single arm meta-analysis. Gastroenterology & Endoscopy. 2, 25–37 (2024).
7 J.J. Manfredi, S.B. Horwitz. Taxol: an antimitotic agent with a new mechanism of action. Pharmacol Ther. 25, 83–125. (1984).
8 R. B. Mokhtari, T.S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das, H. Yeger. Combination therapy in combating cancer. Oncotarget. 8, 38022–38043 (2017).
Posted by buchanle on Thursday, June 26, 2025 in May 2025.
Tags: 5-Fluorouracil, Cell Viability, Combination Chemotherapy, Paclitaxel

