Development of “Parkinson’s-like” disease in C. elegans Using Dextrose and a 6-OHDA Neurotoxin
ABSTRACT
Parkinson’s Disease is a neurodegenerative disease in the substantia nigra of the mesencephalon, a region of the midbrain consisting of dopaminergic neurons that send signals through the spinal cord to the rest of the body. Currently, there are 500,000 patients diagnosed with Parkinson’s Disease in the United States each year. Previous research suggests that neuronal death in this brain region is due to the production of reactive oxygen species (ROS). The neurotoxin 6-OHDA is thought to work via the production of ROS. Caenorhabditis elegans (C. elegans), a type of nonparasitic roundworm, were used to determine if excess dextrose would worsen the damage caused by 6-OHDA. Dextrose had previously been proven to increase the neurotoxicity of 6-OHDA, and this research examines to what extent the neurotoxicity increases. A mobility shift pattern (MSP) assay was used to determine if each of the C. elegans being tested had “Parkinson’s-like” disease. It was determined that as the concentration of dextrose increased, the percent of C. elegans that had “Parkinson’s-like” symptoms rose; dextrose caused a statistically significant increase in neurotoxicity of the 6-OHDA neurotoxin.
INTRODUCTION.
Parkinson’s disease (PD) affects more than 10 million patients worldwide at the moment with the number steadily climbing [1]. Symptoms of the disease include tremors, bradykinesia (slowness of movement), rigidity, and decreased cognitive function [2]. PD is caused by the expansive death of dopaminergic neurons in the substantia nigra which causes little to no dopamine that is sent to the rest of the body causing the above symptoms.
PD pathogenesis seems to be closely related to oxidative stress resulting from generation of reactive oxygen species (ROS) [3]. It has been shown that ROS and oxidative stress contribute to the damage and loss of dopaminergic neurons. [6] 6-Hydroxydopamine (6-OHDA, Figure 1) is a neurotoxin that creates ROS. 6-OHDA creates ROS as monoamine oxidase, an enzyme that accelerates oxidation rates, metabolizes this neurotoxin. ROS metabolize the dopamine produced by the dopaminergic neurons. Thus, the number of dopaminergic neurons is lowered because of the ROS attack of oxidative stress on these neurons. Dopaminergic neurons are neurons which provide the main source of dopamine in the brain. When these neurons can no longer provide dopamine, they degenerate causing a lack of dopamine which can be sent to the rest of the body. This lack of dopamine results in the symptoms mentioned before such as tremors and bradykinesia. The 6-OHDA neurotoxin enters these dopaminergic neurons through a dopamine transporter (DAT). Production of ROS within the dopaminergic neurons eventually leads to their degradation and death [4, 5].
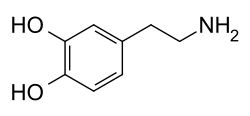
Figure 1. The right compound shows the chemical makeup of the neurotoxin, 6-OHDA while the left compound shows the chemical makeup of dopamine. The neurotoxin lowers the number of dopaminergic neurons by allowing them to enter their membranes through the DAT. The similarities between their chemical makeups show why the DAT allows the neurotoxin to enter the dopaminergic neuron.
Increased ROS production has been linked to the cellular response to high dextrose levels [4]. Our experimental question was to find if excess dextrose would increase the neurotoxicity of 6-OHDA. Our hypothesis was that if C. elegans, a type of non-parasitic roundworm and the subject for our research, was pretreated with a higher percentage of dextrose, they would be more susceptible to the 6-OHDA and not complete the MSP assay as frequently. C. elegans reproduce every three days, and their nervous systems have already been completely mapped out, making them the ideal model organism.
MATERIALS AND METHODS.
C. elegans roundworms were placed on nematode growth medium (NGM) agar plates, on which they lived. E. coli OP-50 accompanied the roundworms on the plates as a food source. We then allowed the roundworms to develop over 72 hours before we began age synchronization. C. elegans roundworms and eggs were placed in a 4% hypochlorite solution for 8 minutes. Once the roundworms have dissolved, the remaining eggs are placed in an M9 buffer for 24 hours for the eggs to hatch. C. elegans were then placed on different treatment plates. Four different types of treatment plates were used: one for a control plate; one on which to test; one treatment plate contained NGM agar, OP-50, and a 2% dextrose solution; and one treatment plate contained NGM agar, OP-50, and a 4% dextrose solution. After 72 hours of the C. elegans living on the treatment plates, they were given 150 μL 2 mM ascorbic acid for the control plate and 150 μL 6-OHDA for each of the other three plates. 10 mM 6-OHDA was used during the first trial, 5 mM 6-OHDA during the second trial, and 2.5 mM 6-OHDA for the final three trials. After one hour, C. elegans treated with the 6-OHDA neurotoxin for each pretreatment are placed on separate NGM agar plates without OP-50. After 24 hours, the mobility shift pattern assay is performed for each plate. To test whether each roundworm has received “Parkinson’s-like” disease, they were given a mobility shift pattern (MSP) assay to complete. The MSP assay is completed when the roundworms can start in a sinusoidal state and return to that same state after being placed in a droplet of liquid. Failure to return to a sinusoidal state suggests their having “Parkinson’s-like” disease.
C. elegans are individually placed on an NGM agar plate with no OP-50. 2 μL S-Basal droplet is added to each worm and removed after 2 minutes with a cleaning wipe. We then recorded whether the C. elegans could return to a sinusoidal state and completing the assay.
RESULTS.
We first used 10 mM 6-OHDA and next 5 mM 6-OHDA which both had small ranges in which percentages could vary. 2.5 mM 6-OHDA however gave a large enough range to see affected areas more clearly. To ensure clearer results, we used 2.5 mM 6-OHDA to distinguish between each of the treatment groups. Unlike the other two concentrations, 2.5 mM 6-OHDA provided a clear 20% divide in the percent of C. elegans that demonstrated “Parkinson’s-like” Disease symptoms between each of the treatment groups. One trial was performed on each of these concentrations while three trials were performed with 2.5 mM 6-OHDA. Higher levels of 6-OHDA resulted in a large percentage of worms with PD-like symptoms. Thus, any exacerbation of the symptoms by excess dextrose could not be detected. 2.5 mM 6-OHDA was utilized as the working concentration to determine the effect of excess dextrose.
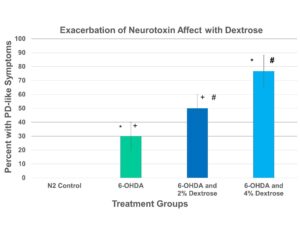
Figure 2 shows the percentage of C. elegans found to have “Parkinson’s-like” disease after given 2.5 mM 6-OHDA. Each of the treatment groups is both statistically independent and significant on each other. This statistical significance is shown in the linear growth of neurotoxicity as the percentage of dextrose added is increased.
Figure 2. A significant treatment effect was determined between groups utilizing an ANOVA (F = 75.4 , p < 0.001, n = 110). A post hoc Tukey analysis indicated a significant increase in PD-like symptoms with 6-OHDA. Excess dextrose significantly increased the percentage of worms with PD-like symptoms. The addition symbol(+) in Table 2 is present to demonstrate a statistical significance between the 6-OHDA and the 6-OHDA and 2% Dextrose treatment groups. The hashtag symbol(#) demonstrates the same relationship between the 6-OHDA and 2% Dextrose and the 6-OHDA and 4% Dextrose treatment groups as well as the star(*) demonstrates that relationship between the 6-OHDA and the 6-OHDA and 4% Dextrose treatment groups.
DISCUSSION.
All 6-OHDA concentrations produced a treatment effect with a greater percentage of C. elegans with PD-like symptoms. Both the 2% dextrose and the 4% dextrose treatment groups increased significantly in neurotoxicity from solely 6-OHDA [Table 1]. Each of our treatment groups were statistically significant to one another showing the positive, linear trend of C. elegans having an increased neurotoxicity due to increased amounts of dextrose. This data analysis shows how our hypothesis is supported as the C. elegans seemingly had higher susceptibility to PD-like disease as we pretreated them with a higher percentage of dextrose which caused an increase in neurotoxicity. One path for future research is testing with lower concentrations of the neurotoxin 6-OHDA. This extension would allow us to see the extent to which the neurotoxin 6-OHDA affects the neurotoxicity and “Parkinson’s-like” symptoms of C. elegans. Another path for further research would be to measure the differences in ROS damage between groups in cell culture or to experiment with the quantity of dextrose with which the C. elegans are treated. Changing the amount of dextrose with which we treat the C. elegans would show how much the dextrose impacts the increase in neurotoxicity compared to how much the 6-OHDA impacts the neurotoxicity of C. elegans. Previously, research was conducted on the impact dextrose had on the C. elegans as they were given “Parkinson’s-like” disease with OP-50 [6]. Our research builds upon their research as we examined the concentration at which the C. elegans should be evaluated and the impact 2.5 mM the 6-OHDA neurotoxin had on the C. elegans [6].
Table 1. The series of tests to determine the concentration of 6-OHDA to use is shown. The percentages shown in the table demonstrate the percent of C. elegans that demonstrated “Parkinson’s-like Disease” symptoms.
| Concentration of 6-OHDA | 6-OHDA Treatment | 6-OHDA and 2% Dextrose Treatment | 6-OHDA and 4% Dextrose Treatment |
| 2.5 mM | 30% | 50% | 75% |
| 5 mM | 60% | 80% | 80% |
| 10 mM | 80% | 90% | 100% |
ACKNOWLEDGMENTS.
We would like to thank Belmont University, President Bob Fisher, and Dr. Thomas Burns for funding this research. We would also like to thank Dean Spence for executing the research program.
REFERENCES
- American Parkinson’s Disease Association, (APDA), “Understanding the Basics of Parkinson’s Disease” (APDA, 2004; www.apdaparkinson.org/).
- Brazier, Yvette, Medical News Today, “Parkinson’s Disease and its Causes”, www.medicalnewstoday.com/articles/323396.php (2018).
- Nass, Richard, et al., Neurotoxin-induced Degeneration of Dopamine Neurons in Caenorhabditis elegans. Proceedings of the National Academy of Sciences 36, 1-6 (2001).
- Manoharan, Shanmugam, Guillemin, Gilles et al., The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxidative Medicine and Cellular Longevity 2016, 1-15 (2016).
- Shamsuddin, Haleema, poster presented at the 4th Annual Belmont University Poster Symposium, Nashville, TN, “Investigating the Effects of a Hyperglycemic Diet on The Mobility Behavior of Caenorhabditis elegans with Parkinson’s Disease” (2018).
- Guo JD, Zhao X et al., Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). International Journal of Molecular Medicine 41, 1817-1825 (2018).
Posted by John Lee on Wednesday, December 23, 2020 in May 2020.
Tags: C. elegans, Dextrose, Neurotoxin, Parkinson’s disease