Crossing the blood-brain barrier: recent advances in nanoparticle strategies for drug delivery to the CNS
ABSTRACT
The brain is one of the most important organs in the human body. The blood-brain barrier (BBB) is an essential protection mechanism for the central nervous system (CNS), preventing pathogens and foreign substances from reaching the brain. Consequently, the BBB represents a substantial obstacle to drug delivery. In the last few decades, researchers have explored different strategies to assist drugs to cross the BBB. Nanoparticles are emerging as an effective and non-invasive system to treat nervous system disorders. Here, we review the latest advances in BBB-crossing nanoparticles, with an emphasis on their clinical translation.
THE NEUROVASCULAR UNIT
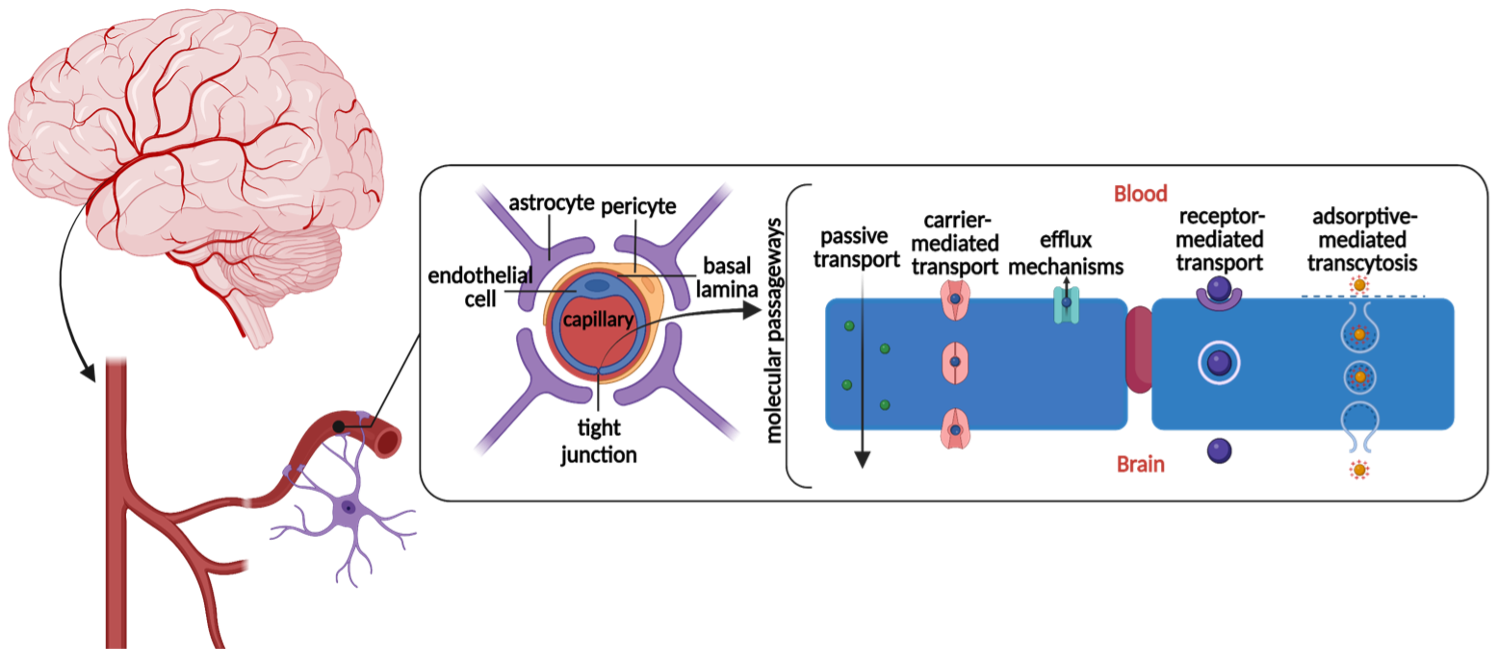
The brain is the most complex organ in the human body, distinguished by its high specialization, structural and functional hierarchy, and constant metabolic demand [1]. Several mechanisms ensure the continuous and controlled transport of oxygen and nutrients to the brain, through an organized group of cells of both vascular and neural origin called the neurovascular unit (NVU) [1]. The NVU comprehend several main components that help regulate the passing of molecules to the brain. These include endothelial cells, pericytes, astrocytes, and microglia (Figure 1). Understanding the NVU components and brain physiology is fundamental for the development of delivery systems capable of directly targeting the central nervous system.
Figure 1. Components of the NVU. Biological molecules can cross the BBB using different passageways. The figure shows the most common pathways of transportation. (Created using Biorender.com)
Blood–brain barrier. The blood-brain barrier (BBB) describes the network of blood vessels that protects the functional tissue (parenchyma) of the brain from harmful substances [2]. It is a highly regulated structure that only allows essential substances to pass. The cerebral capillaries of the BBB are lined with highly packed, flat, endothelial cells that form tight junctions to seal pathways between cells [2]. Endothelial cells present a lack of fenestrations (openings) on the membrane, thereby preventing the non-specific passage of polar molecules between the blood and the brain. Moreover, endothelial cells prevent non-specific pinocytotic events. All of these features selectively control the passage of nutrients while preventing the influx of toxins [2]. Endothelial cells are encircled by the basal lamina which is a connective tissue surrounding the blood vessel. Pericytes connect with the basal lamina on the other side [2].
Pericytes. Pericytes support the structure of the microvessel and signal endothelial cells to regulate the growth and permeability of the cell membrane [2]. Pericytes control BBB permeability via its contractile fibres that regulate capillary diameter, thereby controlling cerebral blood flow [2]. Pericytes also act as a line of defence as they can eliminate harmful microorganisms that cross into the brain through endocytosis [2].
Astrocytes. As a type of glial cell, astrocytes provide structural properties to the BBB. They form contacts with the entire vascular system to regulate oxygen and glucose transport and help blood components to cross the BBB [2]. Astrocytes also regulate water and ion concentrations and control the clearance of neurotransmitters, multiplication of stem cells, and synapses number; they act as an additional barrier before compounds can enter the brain [3] (Figure 1).
PASSAGEWAYS: HOW MOLECULES CAN PASS THROUGH THE BBB
The BBB functions as a protective network in the brain but essential molecules such as oxygen, water, and glucose must be able to cross the BBB to provide nutrients to brain cells. There are several ways that these molecules cross the BBB (Figure 1).
Passive Transport. Small lipophilic molecules soluble in the hydrophobic core of the cell membrane can cross the BBB through passive transport [3]. This mechanism does not require energy for the transfer of molecules through the barrier; it is a diffusion process that enables molecules to cross. Passive transport is not highly controlled by the components of the BBB, thus only molecules that fulfill specific requirements can use it. An example is given by molecules with a molecular weight of less than 400 Da and forming less than 8 hydrogen bonds [2].
Receptor–Mediated Transcytosis. This method of transportation takes advantage of overexpressed receptors on the BBB that help larger molecules such as insulin, leptin, and transferrin to cross the barrier [4]. External agents such as nanoparticle systems can be engineered with these molecules that can match the target receptor [4]. The receptors are then able to recognize and bind with the ligand attached to the nanoparticle and create a receptor-ligand complex which is then internalized through endocytosis [5].
Adsorptive-Mediated Transcytosis. Adsorptive-mediated transcytosis is a type of transport that leverages electrostatic interactions between the negatively charged sialoglycoproteins of glycocalyx and cationic molecules [3]. The attraction between the two charges enables certain molecules such as peptides to cross the BBB. Nanoparticles can be functionalized by attaching cationic ligands to the surface of the particle. Nevertheless, this is not an efficient way of targeting certain cells/areas of the brain since nanoparticles interact with all negatively charged cell membranes and there are low rates of this way of vesicle transport [4].
Carrier–Mediated Transport. Carrier-mediated transport is usually used by endogenous molecules such as glucose and amino acids in facilitated or active diffusion [6]. In the former, molecule-specific transporters go down the concentration gradient, while in the active diffusion transporters go against it [6]. Since carrier-mediated transport allows molecules to both enter and exit the brain, it is not the most useful mechanism for nanoparticles to enter as they can be easily released back out through efflux mechanisms that control waste removal from the brain [6].
WHAT ARE NANOPARTICLES & HOW DO THEY WORK?
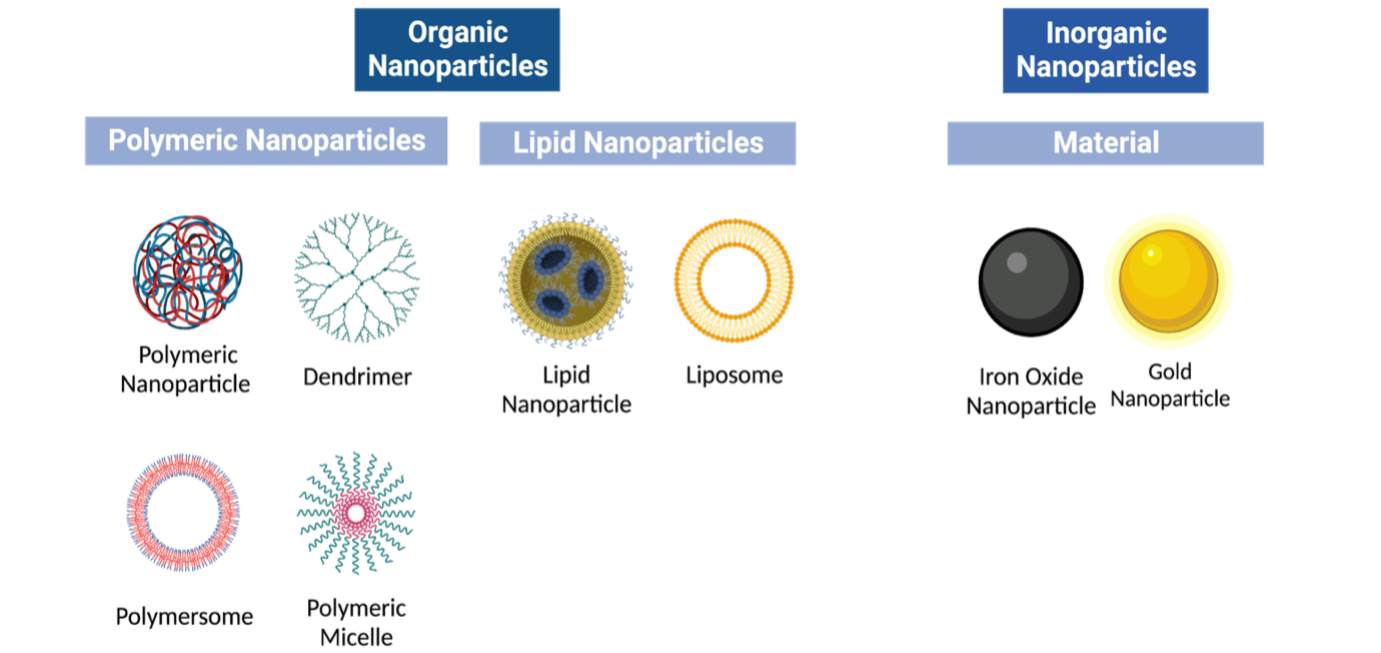
Nanoparticles are organic or synthetic molecules on the nanoscopic scale used for targeted drug delivery and disease diagnosis [7]. They are also used to overcome biological barriers, such as crossing the BBB. Nanoparticles are organized into different classes based on their different physicochemical characteristics (Figure 2).
Figure 2. Nanoparticle classification. Organic and inorganic nanoparticles present different sub-classes of varied material and structure with some of the most common illustrated here. Many different sub-classes of nanoparticles accommodate for different drug deliveries and body conditions. (Created using Biorender.com)
Lipid–based nanoparticles. Lipid-based nanoparticles are spherical entities with at least one lipid bilayer surrounding an internal aqueous chamber [7]. Advantages of lipid-based nanoparticles include being highly biocompatible and the ability to encapsulate larger-sized cargo [8-9]. A subset of lipid-based nanoparticles are liposomes and lipid nanoparticles. Liposomes are composed of phospholipids that can form multilamellar vesicular structures [7]. This allows them to be able to carry a wide range of cargo as they can trap hydrophilic and lipophilic compounds in the same system [10]. However, liposomes can be quickly destroyed by the immune system through the phagocytic cells from the reticuloendothelial system [7,11]. Some classes of lipid nanoparticles form micellar structures with a non-polar core and cationic or ionizable lipids [12]. Ionizable lipids have a near-neutral charge at physiological pH but become positively charged in acidic environments such as endosomal compartments, allowing the endosomal to escape during intracellular delivery [7]. Nevertheless, lipid nanoparticles are limited by their low drug-loading efficiency and biodistribution, being highly uptake by the liver and spleen [13].
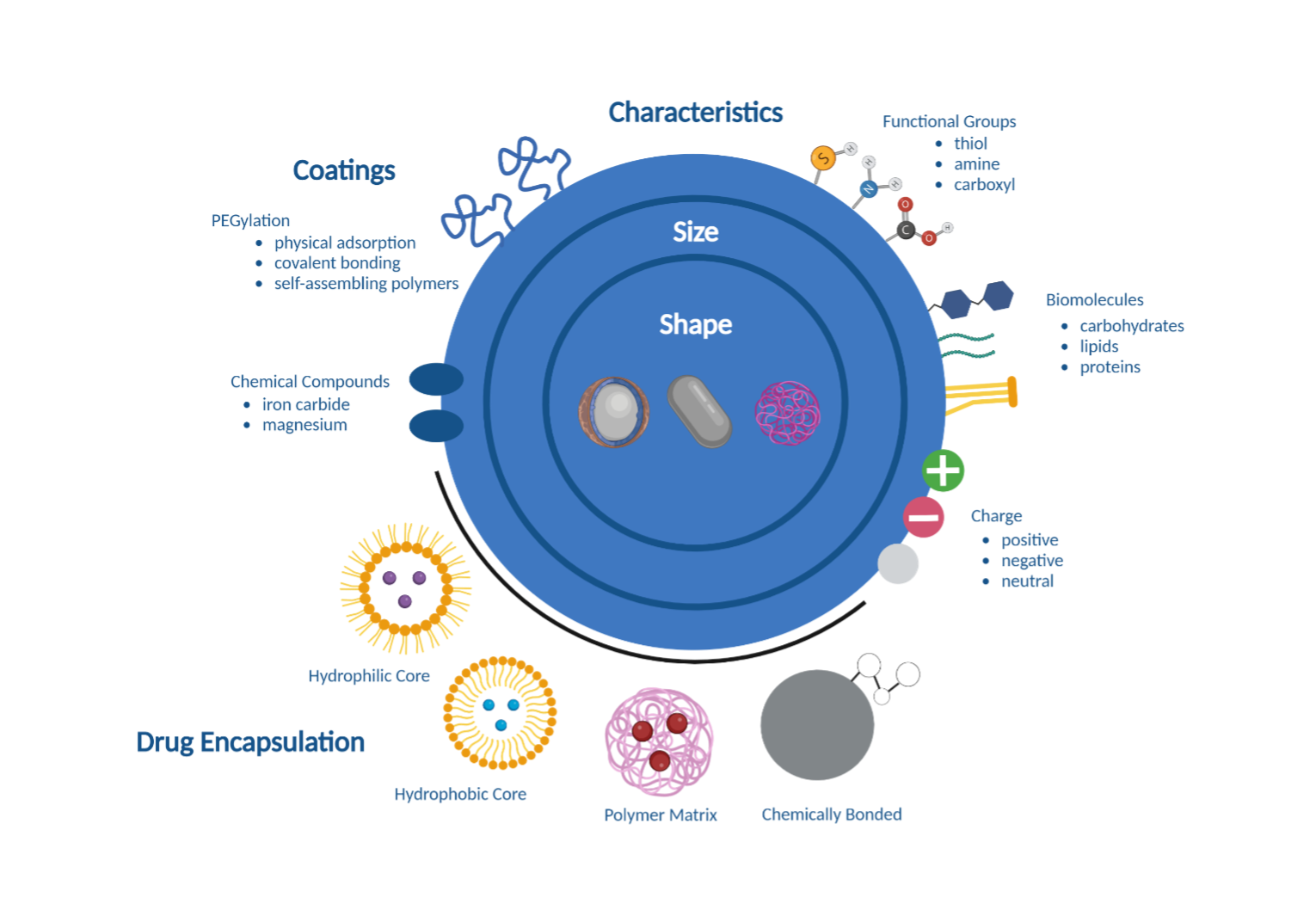
Polymeric nanoparticles. Polymeric nanoparticles are created from natural or synthetic materials that are biocompatible and biodegradable [7] (Figure 3). Drugs delivered by polymeric nanoparticles are either confined in the nanoparticle core, contained in the polymer matrix, bound to the nanoparticle surface, or chemically bonded to the polymer [7]. Their unique structure allows more variety of compounds that can be simultaneously delivered. For example, compounds that are hydrophobic, hydrophilic, and/or of different molecular weights can be transported at the same time, making them ideal for the delivery of macromolecules, proteins, and vaccines [7]. However, polymeric nanoparticles have a higher risk of particle aggregation and toxicity in the body [7].
Figure 3. Nanoparticles for biomedical applications. Different characteristics (size, shape, charge) combined with drug encapsulation and different coatings, are important for nanoparticle interaction with biological systems. (Created using Biorender.com)
Inorganic Nanoparticles. Inorganic nanoparticles have a wide variety of structural characteristics such as size and shape as well as physical, electrical, and radioactive based on the material used [7]. Most inorganic nanoparticles are biocompatible but there are concerns about toxicity especially amongst metal-based nanoparticles due to their low solubility [7].
Gold Nanoparticles. A common material used for creating inorganic nanoparticles is gold (Au). Gold nanoparticles come in many structural forms including nanospheres, nanorods, nanoshells, and nanocages [7]. It is also easily functionalized allowing them to have greater delivery capabilities [7].
Iron Oxide Nanoparticles. Iron oxide presents very interesting proprieties such as superparamagnetism, large surface area, and low toxicity [7]. Nevertheless, those nanoparticles can easily agglomerate and be highly reactive, especially with oxidizing agents [7]. To avoid this, iron oxide nanoparticles can be covered with a coating such as magnesium or iron carbide to make them less reactive and still retain their original properties [7].
CHALLENGES WITH CROSSING THE BBB
The introduction of nanoparticles to the body can be challenging. Particles can be recognized by the immune system and their behaviour under certain conditions can impact their effectiveness. High shear force, enzyme absorbance, rapid clearance of nanoparticles, and their swift secretion must be overcome. The presence of natural barriers such as the BBB prevents their diffusion in many compartments. Nevertheless, there are methods to overcome these limitations. For example, nanoparticles can be coated with materials that are recognized by the body. The most commonly used nanoparticle coating is polyethylene glycol. Polyethylene glycol (PEG) is a polyether coating that allows nanoparticles escape from the immune system and improves their solubility in body fluids [14]. PEG is attached to nanoparticles through physical adsorption, chemical bonding, or conjugation with hydrophobic molecules that can create aqueous self-assembled PEGylated nanoparticles. Once PEG is attached to a hydrophobic polymer or lipid it creates a shield between the nanoparticle and its surrounding by minimizing protein adsorption while in circulation by acting as a non-fouling surface [14].
However, researchers have found that exposure to more than 5 mol% of PEG can result in the body creating anti-PEG antibodies that attach to the nanoparticle and are recognized by macrophages resulting in their liver accumulation [14]. This could lead to toxicity in vivo.
NANOPARTICLE SYSTEMS IN CLINICAL TRIALS
In this paper, we analyzed a few completed case studies of clinical trials that involved the use of nanoparticles to cross the BBB. Overall, the results from Table 1 suggests that a majority of nanoparticle research about crossing the BBB is still in phase 1 of clinical trials. However, there have been a few phase 2 trials of nanoparticles which will be the focus of this discussion. In phase 2 clinical trial carried out by Nagpal, S. et al, the chemotherapeutic agent irinotecan was linked to a polyethylene glycol polymer through a biodegradable link to create NKTR-102. Irinotecan is then released from NKTR-102 and metabolized to cause damage to DNA through topoisomerase I inhibition. The half-life of the compound increased from 2 to 50 days, nevertheless due to its large size it is not able to easily pass back out of vascular membranes such as the BBB and can accumulate in higher concentrations around tumor areas of the brain. Only two patients resulted to show toxicity concerns, and there were no changes to the safety profile established in phase 1. In phase 2 clinical trials carried out by Clene Nanomedicine, CNM-Au8 was tested on patients with Parkinson’s disease. CNM-Au8 are clean-surfaced catalytically active gold nanocrystals, 13nm in diameter, in an oral suspension, which are often used to increase the absorption rate and bioavailability of poorly soluble drugs [28-29]. In results released by Clene Nanomedicine, CNM-Au8 successfully decreased the concentration of free radicals by turning them into oxygen and water, increased NAD+ from NADH to stimulate cellular respiration, and lowered the levels of misfolded proteins by reducing oxidative stress. Along with drug delivery for patients with Parkinson’s disease, CNM-Au8 has also been tested on patients with multiple sclerosis and amyotrophic lateral sclerosis which highlights the versatility of this nanoparticle formulation. Overall, during this phase of clinical trials, the clean-surface gold nanoparticle and target molecule interactions were successful and passed safety standards [28]. Although these clinical trials have been successful, they are still in their early stages of development. Nonetheless, given that many nanoparticle investigations are still in their in vitro and in vivo studies, reaching the clinical trial stage can be considered a form of success. The nanoparticle size is one of the main reasons that prevent nanomedicine to reach an advanced clinical stage. Most of them are too large to be taken in by transcytosis vesicles, and once inside the brain, the extracellular space is extremely dense, making movement and targeting difficult for nanoparticles [4]. Nanoparticles must fit into the extracellular spaces which range from 50 nm to 100 nm [4]. Many constraints exist when synthesizing nanoparticles for brain-targeted delivery systems slowing the progression of current research.
| Table 1. Nanoparticles in clinical trials. | ||||
| Nanoparticle | Clinical Trial
Reference |
Disease | Stage | Reference |
| Gold nanoparticle | NCT03020017 | Gliosarcoma
Recurrent Glioblastoma |
Early Phase 1 | [15], [16] |
| CPT-11 (NL CPT-11) | NCT00734682 | Recurrent High-Grade Gliomas | Phase 1 | [17], [18] |
| Irinotecan molecules attached to a polyethylene glycol (PEG) polymer | NCT01663012 | Anaplastic Astrocytomas
Anaplastic Oligodendrogliomas Glioblastomas |
Phase 2 | [19], [20] |
| Irinotecan Liposome | NCT03086616 | Diffuse Intrinsic Pontine Glioma | Phase 1 | [21] |
| AGuIX Gadolinium Based Nanoparticles (NANO-RAD) | NCT02820454 | Brain Metastases | Phase 1 | [22], [23] |
| Nanocells | NCT02766699 | Glioblastoma
Astrocytoma, Grade IV |
Phase 1 | [24], [25] |
| Doxorubicin-loaded Anti-EGFR-immunoliposomes (C225-ILs-dox) | NCT03603379 | Glioblastoma | Phase 1 | [26] |
| Gold Nanocrystals | NCT03815916 | Parkinson’s Disease | Phase 2 | [27] |
CONCLUSIONS
In conclusion, it is important to understand the biology of the NVU to develop delivery systems for CNS therapy. A better understanding of the relationship between nanoparticles and the body will help create materials that can avoid an immune response with the help of different physical and chemical modifications. However, this research is still just a small portion of the way to finding cures for neurodegenerative diseases. Crossing the BBB requires nanoparticles to be modified in a specific way, which needs to match the properties of the drug being delivered to be compatible with each other. Because of these factors, nanoparticles often have to go through multiple rounds of pre-clinical and clinical testing before getting approved. Nanoparticle research and applications to the brain are still quite new. Therefore, this paper provided a basic background of the biology and gathered some of the most promising research to provide insight into the progress toward finding a treatment for CNS diseases.
ACKNOWLEDGMENTS
Thank you for the guidance of Francesca Melle from the University of Cambridge and the team at Lumiere Education.
REFERENCES
- V. Muoio, P. B. Persson, M. M. Sendeski, The neurovascular unit – concept review. Acta physiologica, 210(4), 790-798 (2014).
- H. Kadry, B. Noorani, L. Cucullo, A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 17, 69 (2020).
- A. D. Wong, M. Ye, A. F. Levy, J. D. Rothstein, D. E. Bergles, P. C. Searson, The blood-brain barrier: an engineering perspective. Front. Neuroeng. 6, 7 (2013).
- R. G. R. Pinheiro, A. J. Coutinho, M. Pinheiro, A. R. Neves, Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int. J. Mol. Sci. 22 (2021), doi:10.3390/ijms222111654.
- W. Stillwell, “Chapter 17 – Moving Components Through the Cell: Membrane Trafficking” in An Introduction to Biological Membranes (Second Edition), W. Stillwell, Ed. (Elsevier, 2016), pp. 369–379.
- S. Reddy, K. Tatiparti, S. Sau, A. K. Iyer, Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov. Today. 26, 1944–1952 (2021).
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas, R. Langer, Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
- A.-C. Genix, J. Oberdisse, Nanoparticle self-assembly: from interactions in suspension to polymer nanocomposites. Soft Matter. 14, 5161–5179 (2018).
- L. Sercombe, T. Veerati, F. Moheimani, S. Y. Wu, A. K. Sood, S. Hua, Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 6, 286 (2015).
- M. Sarfraz, A. Afzal, T. Yang, Y. Gai, S. M. Raza, M. W. Khan, Y. Cheng, X. Ma, G. Xiang, Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics. 10 (2018).
- R. Alyautdin, I. Khalin, M. I. Nafeeza, M. H. Haron, D. Kuznetsov, Nanoscale drug delivery systems and the blood-brain barrier. Int. J. Nanomedicine. 9, 795–811 (2014).
- A. K. K. Leung, Y. Y. C. Tam, S. Chen, I. M. Hafez, P. R. Cullis, Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B. 119, 8698–8706 (2015).
- O. S. Fenton, K. N. Olafson, P. S. Pillai, M. J. Mitchell, R. Langer, Advances in Biomaterials for Drug Delivery. Adv. Mater., e1705328 (2018).
- L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng, Q. Wang, Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 13, 10748–10764 (2021).
- NU-0129 in Treating Patients with Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery, (available at https://www.clinicaltrials.gov/ct2/show/NCT03020017?term=NCT03020017&draw=2&rank=1)
- P. Kumthekar, C. H. Ko, T. Paunesku, K. Dixit, A. M. Sonabend, O. Bloch, M. Tate, M. Schwartz, L. Zuckerman, R. Lezon, R. V. Lukas, B. Jovanovic, K. McCortney, H. Colman, S. Chen, B. Lai, O. Antipova, J. Deng, L. Li, S. Tommasini-Ghelfi, L. A. Hurley, D. Unruh, N. V. Sharma, M. Kandpal, F. M. Kouri, R. V. Davuluri, D. J. Brat, M. Muzzio, M. Glass, V. Vijayakumar, J. Heidel, F. J. Giles, A. K. Adams, C. D. James, G. E. Woloschak, C. Horbinski, A. H. Stegh, A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 13 (2021).
- A Phase I Trial of Nanoliposomal CPT-11 (NL CPT-11) in Patients with Recurrent High-Grade Gliomas, (available at https://www.clinicaltrials.gov/ct2/show/NCT00734682?term=NCT00734682&draw=2&rank=1)
- A. S. Wadajkar, J. G. Dancy, D. S. Hersh, P. Anastasiadis, N. L. Tran, G. F. Woodworth, J. A. Winkles, A. J. Kim, Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9 (2017).
- Phase II NKTR-102 In Bevacizumab-Resistant High Grade Glioma, (available at https://www.clinicaltrials.gov/ct2/show/study/NCT01663012?term=NCT01663012&draw=2&rank=1).
- S. Nagpal, C. K. Recht, S. Bertrand, R. P. Thomas, A. Ajlan, J. Pena, M. Gershon, G. Coffey, P. L. Kunz, G. Li, L. D. Recht, Phase II pilot study of single-agent etirinotecan pegol (NKTR-102) in bevacizumab-resistant high grade glioma. J. Neurooncol. 123, 277–282 (2015).
- CED With Irinotecan Liposome Injection Using Real Time Imaging in Children with Diffuse Intrinsic Pontine Glioma (DIPG) (PNOC 009), (available at https://www.clinicaltrials.gov/ct2/show/NCT03086616? term=NCT03086616&draw=2&rank=1)
- Radiosensitization of Multiple Brain Metastases Using AGuIX Gadolinium Based Nanoparticles – Full Text View – ClinicalTrials.gov, (available at https://www.clinicaltrials.gov/ct2/show/NCT02820454? term=NCT02820454&draw=2&rank=1)
- C. Verry, S. Dufort, J. Villa, M. Gavard, C. Iriart, S. Grand, J. Charles, B. Chovelon, J.-L. Cracowski, J.-L. Quesada, C. Mendoza, L. Sancey, A. Lehmann, F. Jover, J.-Y. Giraud, F. Lux, Y. Crémillieux, S. McMahon, P. J. Pauwels, D. Cagney, R. Berbeco, A. Aizer, E. Deutsch, M. Loeffler, G. Le Duc, O. Tillement, J. Balosso, Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 160, 159–165 (2021).
- A Study to Evaluate the Safety, Tolerability and Immunogenicity of EGFR(V)-EDV-Dox in Subjects with Recurrent Glioblastoma Multiforme (GBM) – Full Text View – ClinicalTrials.gov, (available at https://www.clinicaltrials.gov/ct2/show/NCT02766699?term=NCT02766699&draw=2&rank=1)
- S. M. Sagnella, L. Yang, G. E. Stubbs, E. Boslem, E. Martino-Echarri, K. Smolarczyk, S. L. Pattison, N. Vanegas, E. St Clair, S. Clarke, J. Boockvar, J. A. MacDiarmid, H. Brahmbhatt, Cyto-Immuno-Therapy for Cancer: A Pathway Elicited by Tumor-Targeted, Cytotoxic Drug-Packaged Bacterially Derived Nanocells. Cancer Cell. 37, 354–370.e7 (2020).
- Doxorubicin-loaded Anti-EGFR-immunoliposomes (C225-ILs-dox) in High-grade Gliomas – Full Text View – ClinicalTrials.gov, (available at https://clinicaltrials.gov/ct2/show/study/NCT03603379).
- 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Parkinson’s Disease – Full Text View – ClinicalTrials.Gov, (available at https://clinicaltrials.gov/ct2/show/study/NCT03815916).
- Clene inc, (available at https://clene.com/).
- V. R. Patel, Y. K. Agrawal, Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2, 81–87 (2011).
Posted by John Lee on Tuesday, May 30, 2023 in May 2023.
Tags: Blood Brain Barrier, clinical trials, drug delivery, Nanoparticles