Casting Polyvinylidene Difluoride on Graphene for Nanoscale Filtration Systems
ABSTRACT
Filtering molecules based on size is something that chemical engineers have been striving to achieve at a scalable level. Filtering molecules allows for desalinization, bioprocessing, harnessing energy in fuel cells, and filtering out aerosols. Graphene is an atomically thin material that can sustain nano-sized pores that allow for certain molecules to pass through, while keeping others out. Graphene is a delicate film that requires additional support for membrane applications. Here we show that polyvinylidene difluoride (PVDF) provides a stable and supportive structure with uniform pores to support graphene. PVDF has the potential to be a beneficial polymer for fabricating graphene membrane. The goal of this project is to further study PVDF when cast upon graphene, optimizing the best casting conditions and producing consistent, uniform pores.
INTRODUCTION.
Filtering molecules using scalable processes allows for a wide variety of applications, including but not limited to desalinization, bio separations and processing, harnessing energy, and filtering aerosol [1]. These filtration systems can be made out of two-dimensional materials that have nanoscale pores [2]. The pore sizes can be altered to allow smaller molecules to pass through and no unwanted molecules pass through. The membranes act like a high functioning filtration system, where you can customize what flows through.
These systems can be constructed out of a hexagonal carbon structure known as graphene. Graphene is a two-dimensional material that can be grown through a process called Chemical Vapor Deposition (CVD), where chemical precursors are deposited onto copper. The copper, however, does not possess the porous structure necessary for filtering, so it must later be removed, and to act as a filtering system, the graphene must be placed on a supportive porous membrane.
Graphene is delicate and requires structural support. It uses copper for stability during the CVD growth process and the copper also acts as a catalyst for the reaction, but once it is grown, the graphene needs an additional layer of structure. Polymers make for excellent structural support, and they also can be conditioned to have optimal pore sizes. Up until recently, a commonly used polymer has been Polyethersulfone (PES), because the casting process is quick, and the polymer is resistant to a lot of different chemicals. However, more recent studies have shown that polyvinylidene difluoride (PVDF) provides both more chemical and thermal resistance [3] [4].
Several researchers have looked at different parts of the PVDF casting process and adjusted the methodology behind it to make the polymer more stable and the pores more uniform. Many looked at the phase inversion step in casting, which changes the polymer from liquid to solid, adjusting the solvent that membrane is placed in to set [5]. The goal with each of these adjustments is to determine which casting conditions produce the most optimal pore qualities. To achieve the best pore qualities, pore sizes across the membrane must be consistent, and porosity, or pore coverage, must cover the entire membrane.
Since PVDF has proven to be an adequate casting material, it makes sense to continue testing different casting variables in order to find the best solution. The goal of this research project is to identify the best possible conditions for PVDF casting, by testing a variety of antisolvents during phase inversion and then analyzing the pore sizes and pore coverages using a scanning electron microscope (SEM).
PVDF castings will likely provide a more reliable structure, because PVDF itself has a higher level of chemical and thermal resistance than PES [3]. Water may prove to be a better solvent than isopropyl alcohol and will produce smaller and more concentrated pores across the membranes. The IPA solvent bath will likely react with the IPA present in the PVDF solution and form non-circular pores, whereas the water will not react with the solution at all and will form consistent, circular pores [5]. The consistent pores will make water the better anti-solvent used during the phase inversion.
MATERIALS AND METHODS.
The following methods were adapted from the Kidambi Lab.
Making PVDF Solution.
PVDF Solution was prepared by mixing 0.5g PVDF (10 wt%) in 4.4g of N-Methyl-2-pyrrolidone (NMP, 88 wt%) and 0.1g of Isopropyl Alcohol (IPA, 2 wt%). The solution was then baked at 40 °C and degassed overnight. It was then stored in a light-proof container at room temperature. See Figure S1 for schematic.
Casting PVDF Solution using Tape as a Channel.
A copper sheet with general graphene deposited on top was cut to measure about 2.5 by 2.5 cm, then was taped onto an aluminum plate, with a piece of scotch tape on the top and bottom edges of the sheet, and then three layers of tape on each side (each tape about 50 µm thick). The PVDF solution was then cast via pipette atop the copper sheet and rolled using a glass cell culture tube. Rolling the solution provided an even coverage of PVDF on top of the graphene.
Phase Inversion.
Phase inversion is the process that removes the solvent from a liquid polymer and leaves behind a porous structure. The PVDF casting was placed immediately in a solvent bath for four hours to complete phase inversion. In this experiment, different solvents (distilled water or isopropyl alcohol) were used for each sample to test the how they affected the pores of the membranes.
Etching Copper Off the PVDF Layer.
Once removed from the bath, the copper was cut out of the tape and allowed to dry. The copper was then etched away using ammonium persulfate (APS). See Figure S2 for schematic.
Imaging.
The casting was attached to a glass slide using carbon tape with the graphene side facing up. The slide was inserted into the Zeiss Merlin Scanning Electron Microscope (SEM) and images were collected, first looking at the top of the sample, then looking at the cross section, or edge. A 2 kV beam power and a 90-pA beam current were used to capture the images. The top of the sample shows the porosity of the sample, looking at both the number of pores and their size. The cross section shows the thickness of the membrane from a side view, in order to get a clear measurement.
Using ImageJ, the images’ porosity and average pore size were determined. Porosity was found using the threshold tool, toggling the percentage cover until it met most of the pores. The average pore size was found by setting the scale of the image and drawing lines across the diameter of the pores. Several measurements were taken to accumulate an average. Each cross section was measured by setting the scale and drawing lines across the PVDF layer in the image. See Figures S3 and S4 for an explanation within the software.
RESULTS.
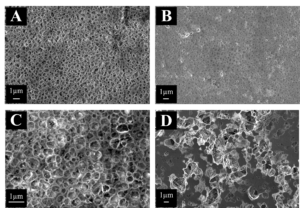
When comparing PES and PVDF membranes, pore size, shape, and coverage were considered. Figure 1 shows SEM images of each polymer. From viewing the image itself, it can be stated that PVDF’s pores appear larger than the PES pores. The surface coverage of PVDF pores also appears greater than that of PES. The Figure also shows the comparison between a water phase inversion and an IPA phase inversion. From simply looking at Images C and D, there are obvious differences in the shapes and structures of the pores. The water’s pores are much more circular than those soaked in IPA. IPA had much larger pore sizes, but much smaller porosity.
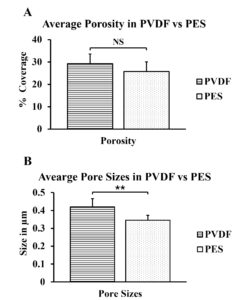
High porosity is important as it is the main mode of transport through the membrane. Figure 2 compares the average porosity of both PVDF and PES. The graph does not depict a drastic enough difference between the two polymers, so a t-test was run to determine if they were, in fact, significantly different. The resulting p-value was 0.1073, which is greater than the significance rate of 0.05; showing that there is no significant difference between the porosity of PVDF and PES.
This research, after determining that PVDF provided a better porosity than PES, sought to find better casting methods to provide higher porosity. After the polymer is cast, it is placed in an anti-solvent bath for several hours to essentially cure the polymer to the membrane it is cast upon. Here, PVDF is tested when cured in water and in isopropyl alcohol, or IPA.
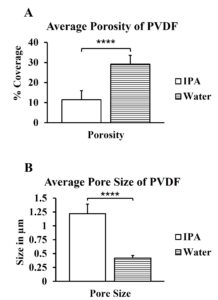
The porosity generated by the IPA and water baths were significantly different from each other, as were the pore sizes between the two. The drastic difference between the pore sizes and the initial lack of circular structure in the IPA samples was due to anti solvent precipitation.
DISCUSSION.
PVDF castings are shown to provide more consistent pores than PES, indicating that PVDF is a better casting polymer than PES. Water was shown to be a better anti-solvent than isopropyl alcohol and will produce smaller and more concentrated pores across the membranes.
The porosity coverage and the pore sizes were more consistent in the PVDF castings than in PES castings. The pores were slightly larger in membranes made with PVDF than in PES, and the porosity increased about 5% in PVDF than in PES. This finding supports the original hypothesis that PVDF will prove to be more efficient than PES. The different liquids used during phase inversion also affected the porosity and pore sizes of the membranes. PVDF castings placed in water had a greater porosity coverage than in isopropyl alcohol {Water ~ 29% coverage, IPA ~ 12% coverage}, just like the project’s original hypothesis. The castings placed in isopropyl alcohol also had much larger pores. The isopropyl alcohol casting produced pores nearly 3 times the size of that with water {Water ~ 0.42µm sized pores, IPA ~ 1.22µm sized pores}. The pores were also non-circular, and the membrane appeared to be spongy.
This large difference in size and shape is likely because the PVDF solution used had alcohol as one of its ingredients. The IPA in the bath acts as an anti-solvent that reacts with the IPA in the PVDF solution. Because of the presence of an anti-solvent, the morphology of the pores is both larger and non-circular.
Water exists as an antisolvent for PVDF, which allows the PVDF to fall out of the solution quickly, which creates tighter, more controlled pore formation. When alcohol is added back into the mix, the solubility of the PVDF solution changes [5]. IPA is already in the PVDF solution, so it doesn’t cause the PVDF solution to fall out as quickly, resulting in a spongy membrane and non-circular pores.
Understanding which solvent works as the best method of phase inversion allows more consistency in their castings. The use of water creates pore coverage, with little to no gaps or inconsistencies. The membranes soaked in IPA created larger pores. On the contrary, the pores created by a water-based phase inversion were of a consistent size and shape.
This research was similar to that of J. Heo and Y. Choi’s in 2016 [6]. In their paper on Material Chemistry, looked at how the different liquids affected the membrane’s pores, testing water, methanol, ethanol, and 2-propanol.
This research was able to modify the pore structure of supports for 2D materials for membrane applications. The results of this research determined that polyvinylidene difluoride, or PVDF, and Polyethersulfone, or PES, are interchangeable when it comes to casting on top of two-dimensional materials. There was no significant difference between the two porosities, for the p-value given was greater than 0.05. This shows that both polymers can be used when casting on top of graphene. This research also concluded that during the phase inversion method of casting, water is the most efficient liquid to use. Castings performed in water had a significantly higher porosity, with a p-value of 1.15 x 10E-7. This shows that using water produced a more effective result than using isopropyl alcohol. Knowing both of these things allows researchers to cater their polymer castings on graphene to create the most efficient filtration systems to be used in different systems.
ACKNOWLEDGMENTS.
A huge thank you to everyone in the Kidambi Lab for taking me in for the summer and teaching me so many new things! I loved the lab experience and am so grateful for everyone I met and the opportunities I was given. A huge thank you to my ISR teachers who encouraged me to apply for the REHSS program! I’m so thankful for the opportunity!
SUPPORTING INFORMATION.
Supporting Information includes a more detailed methodology section. It also includes images showing how to use ImageJ.
REFERENCES
1.“Fuel Cells.” Department of Energy, Accessed 14 Nov. 2024. https://www. energy.gov/eere/fuelcells/fuel-cells
- Baroutaji, Ahmad, et al. Additive Manufacturing for Proton Exchange Membrane (PEM) Hydrogen Technologies: Merits, Challenges, and Prospects. International Journal of Hydrogen Energy, 52, 561–84 (2024).
- “Advantages and Applications of PES Filter Membranes – Hawach.” Hawach Scientific, 8 Mar. 2023, https://www.hawachmembrane.com/advantages-and-applications-of-hydrophilic-pes-filter-membranes/
- Hernández, Ali Roberto Ruiz, et al. Chemical Vapor Deposition Synthesis of Graphene on Copper Foils. Graphene – A Wonder Material for Scientists and Engineers, IntechOpen (2022). https://doi.org/10.5772/intechopen.106058.
- Hou, Dandan, et al. Decimeter-Scale Atomically Thin Graphene Membranes for Gas–Liquid Separation. ACS Applied Materials & Interfaces, 13, 10328–35 (2021).
- Heo, J., et al. Controlled Pore Evolution during Phase Inversion from the Combinatorial Non-Solvent Approach: Application to Battery Separators. Journal of Material Chemistry, 4, 9496-9501 (2016).
Posted by buchanle on Thursday, June 19, 2025 in May 2025.
Tags: Graphene, phase inversion, Polyvinylidene Difluoride (PVDF)

