Block-like Membranes by Spin Coating Ring-Opening Metathesis Polymerization for Polar Solvent Dehydration
ABSTRACT
Ethanol dehydration is the process of removing water from ethanol mixtures. This purified ethanol can then be used to manufacture various plastics or used as biofuel. Current methods of ethanol dehydration use large quantities of energy. A new method of separation involves using membranes to remove water contaminants from ethanol. Polyvinyl alcohol (PVA) – the main industrial membrane for polar solvent dehydration – requires a time-consuming preparation to synthesize, deposit, and cross-link. To address this issue, block-like copolymers were synthesized using an innovative new method called spin coating ring-opening metathesis polymerization (scROMP) and modified with various solvents. scROMP allows for synthesis orders of magnitude faster than traditional synthesis techniques. Due to PVA’s lack of olefin groups, it cannot be synthesized using scROMP. This study used poly trans-3,6-endomethylene-1,2,3,6-tetrahydrophthayoyl chloride (pNBDAC) and poly dicyclopentadiene (pDCPD) to create block-like copolymers (polymers consisting of two or monomers) in which the outer pNBDAC block was modified in various solvents, with the intention of mimicking PVA’s selective qualities. Synthesis of block-like copolymers modified with tris was confirmed using IR spectroscopy and contact angle goniometry. Taurine and hydroxamic acid modified membranes did not show complete functionalization. Pervaporation tests were conducted to find ethanol dehydration performance. Of the block-like copolymers, pNBDAC-b-pDCPD (tris) performed the best, with a selectively of 10. This is compared to PVA’s selectivity of 100. Despite showing promise, pNBDAC-b-pDCPD modified membranes are not to the level of effectiveness to replace PVA as a membrane for ethanol dehydration. Future studies can prioritize testing other modifying chemicals on pNBDAC-b-pDCPD membranes or other combinations of monomers.
INTRODUCTION.
This study focuses on the separation of ethanol and water required for the dehydration of ethanol. With the growth of ethanol as a biofuel [1], this chemical separation is becoming more crucial. A commonly used polymer film for ethanol dehydration is polyvinyl alcohol (PVA). PVA is used because of its large amount of hydrophilic hydroxyl groups. This property allows for the selective permeation of water through the PVA membrane. Often, ethanol is used to cut gasoline while still behaving as a combustible fuel. Ethanol also produces less greenhouse gas when burned than gasoline [2]. This separation is also used in the production of ethylene. Ethylene is used in the production of polyethylene, the world’s most common plastic.
Distillation is a method of chemical separation that accounts for about 8% of the US’s annual energy usage. Membrane-based separation via pervaporation – partial vaporization of a chemical mixture – is an emerging, less energy-dependent, alternative to distillation. Membrane-based separation relies on functional groups on the surface of a polymer membrane pulling one chemical component of the mixture more than the other. This form of separation utilizes 90% less energy than distillation [3].
Despite its less energy intensive nature, membrane-based separation is not without its drawbacks. Its largest drawback is the time it takes to synthesize a polymer membrane. Traditional membrane synthesis – or batch synthesis – involves dissolving large amounts of monomer in solvent to begin the polymerization reaction. The mixture then sits for about a day. A commonly used type of polymerization reaction is ring-opening metathesis polymerization (ROMP), which functions by relieving the ring strain within olefin groups to polymerize molecules. The created polymer solution is then deposited onto a spinning substrate to allow for adhesion in a process called spin coating. The entire process can take >24 hours [4-5]. Thus, this synthesis method is inefficient when trying to find an effective combination of modifying solvent and monomers. Additionally, since batch synthesis uses so much solvent, it has environmental repercussions. For instance, dichloromethane (DCM), a ubiquitous solvent in the academic environment, has been shown to have ozone-depleting properties.
To address this shortcoming, the Jennings lab has recently developed a novel technique that combines spin-coating and ROMP into a simultaneous process that has been coined scROMP [6]. With scROMP, membranes can be synthesized, functionalized, and deposited in <5 minutes. Thus, scROMP allows for the rapid testing of different chemistries while using much less environmentally detrimental solvents, enabling more efficient identification of polymers for chemical separation. Since vinyl alcohol has no olefin groups, PVA cannot be synthesized using scROMP [7]. Therefore, this study sought to create a membrane that could be quickly synthesized while retaining the selective qualities of PVA. If a polymer is too hydrophilic, the membrane could draw too much water and end up swelling. Swelling occurs when water fills the gaps between the molecules of a membrane and deforms the shape, causing a decrease in selectivity and structural integrity. To avoid this, we used block-like copolymers. A copolymer is a polymer with two or more monomers, and a block-like copolymer is a copolymer that is synthesized with distinct layers. Trans-3,6-endomethylene-1,2,3,6-tetrahydrophthayoyl chloride (NBDAC), one of the constituent monomers used in this study, had easily modifiable acyl chloride groups that could be exchanged for other functional groups depending on the modifying agent used (tris(hydroxymethyl)aminomethane (tris), taurine, or hydroxamic acid). The other monomer used in this study was dicyclopentadiene (DCPD), which was not easily modifiable, but was used because it provided physical stability to the block copolymer.
MATERIALS AND METHODS.
Silicon Substrate Preparation.
Silicon (100) wafers (University Wafers) were cut into 1.5 cm x 1.5 cm squares using a diamond scribe. The squares were then rinsed with distilled water and ethanol and dried with a nitrogen gas stream prior to use in polymer synthesis.
PAN Support Preparation.
Polyacrylonitrile (PAN) supports were cut into 6 cm x 6 cm squares using scissors and then stored in a 90:10 (v/v) % EtOH/H2O mixture until use in polymer synthesis and pervaporation testing.
Grubb’s 3rd Generation Catalyst Preparation.
Grubb’s 2nd generation catalyst [(H2IMes)(PCy3)(Cl)2Ru=CHPh] (G2) and 3-bromopyridine were used to synthesize Grubb’s 3rd generation catalyst [(H2IMes)(3-Brpy)2(Cl)2Ru=CHPh] (G3) using a previously described method [8-9]. G2 (0.5, 0.59 mmol) and 3-bromopyridine (0.94 g, 5.9 mmol) were added to a 20 mL vial. The solution was stirred for 5 minutes at room temperature. There was an observable color change from red to bright green. 20 mL of pentane was added to the vial and a green solid precipitated. The vial was sealed and placed in a freezer overnight. The solid precipitate was vacuum filtered, washed with 10 mL of pentane four times, and then vacuum dried to yield powdered G3. When it was time for use, the G3 was dissolved in DCM at a concentration of 5 mM to be used in polymer synthesis.
pNBDAC Synthesis.
pNBDAC synthesis occurred via the scROMP process developed in Parkerson et al, 2024. While the PAN or silicon substrate was spinning, 200 μL of dissolved G3 was deposited. The supports spun at 2000 rpm for 30 seconds to allow for evaporation-facilitated deposition of the G3. Immediately following that, 200 µL of neat NBDAC (6.2 M pure liquid) were dispensed onto the spinning substrate at 2500 rpm. NBDAC was selected because of its two easily modifiable acyl chloride groups. The polymerization reaction began immediately, and the substrate was allowed to spin for another 60 seconds before being stopped and stored in a modifying solution. If modified in tris (0.5 M aqueous), the polymer sat for 10 minutes. If modified in hydroxamic acid (1 M aqueous) or taurine (1 M aqueous), it sat overnight. Films were removed from solution prior to characterization. Hydroxamic acid was pH adjusted to 6.2 using triethylamine.
pDCPD-b-pNBDAC Synthesis.
Copolymer synthesis followed the same process as homopolymer synthesis until the G3 was deposited. After that, 200 μL of DCPD (4 M in DCM) were spun on at 2500 rpm for 60s. DCPD was selected due to the stability it offers the copolymer. Following this, DCM was spun on at 2500 rpm to remove unpolymerized DCPD. The substrate was allowed to spin for 30 seconds before a second wash of DCM. 200 μL NBDAC were then spun on at 2500 rpm where it polymerized for 60 seconds. The polymer was then stored in one of the 3 modifying solutions (tris, taurine, or hydroxamic acid). Films were removed from the solution prior to characterization.
Contact angle Goniometry.
Contact angle goniometry measurements were gathered using a Ramé-Hart manual goniometer and ~5 μL droplets of water. The dispensing syringe remained in the droplet to gather advancing contact angles. Contact angle measurements were used to determine the surface composition of the polymer.
KBr Pellet Formation and Transmission IR Spectroscopy.
This method was used to prepare samples for transmission IR spectroscopy to determine bulk polymer composition. Polymer films were scraped from silicon substrate using a razor blade and ground with 0.2 g of KBr in a mortar and pestle. The KBr mixture was then pressed at 7.5 tons for 10 minutes in a Specac Atlas 15T manual hydraulic press to form a glass pellet. Transmission infrared spectroscopy was performed using a Thermo Fisher Scientific Nicolet 6700 FTIR with a DTGS KBr detector. Each sample was scanned 64 times with a blank KBr pellet as a background and analyzed using OMNIC™ software.
Pervaporation Tests.
Pervaporation tests were performed using a Sterlitech Polytetrafluoroethylene Innovator Tangential Flow Cell with 16 cm2 active area where membranes synthesized on PAN supports were placed. The feed solution of 90:10 (v/v) % EtOH/H2O was heated to 60 ℃ and pushed to the polymer interface using a rotary pump. Pervaporation was achieved via a vacuum pump. Permeate was collected and EtOH content was measured using an Atago PAL-34S pocket refractometer. Pervaporation tests were performed to determine selectivity of the polymer membranes.
RESULTS AND DISCUSSION.
Membrane Swelling.
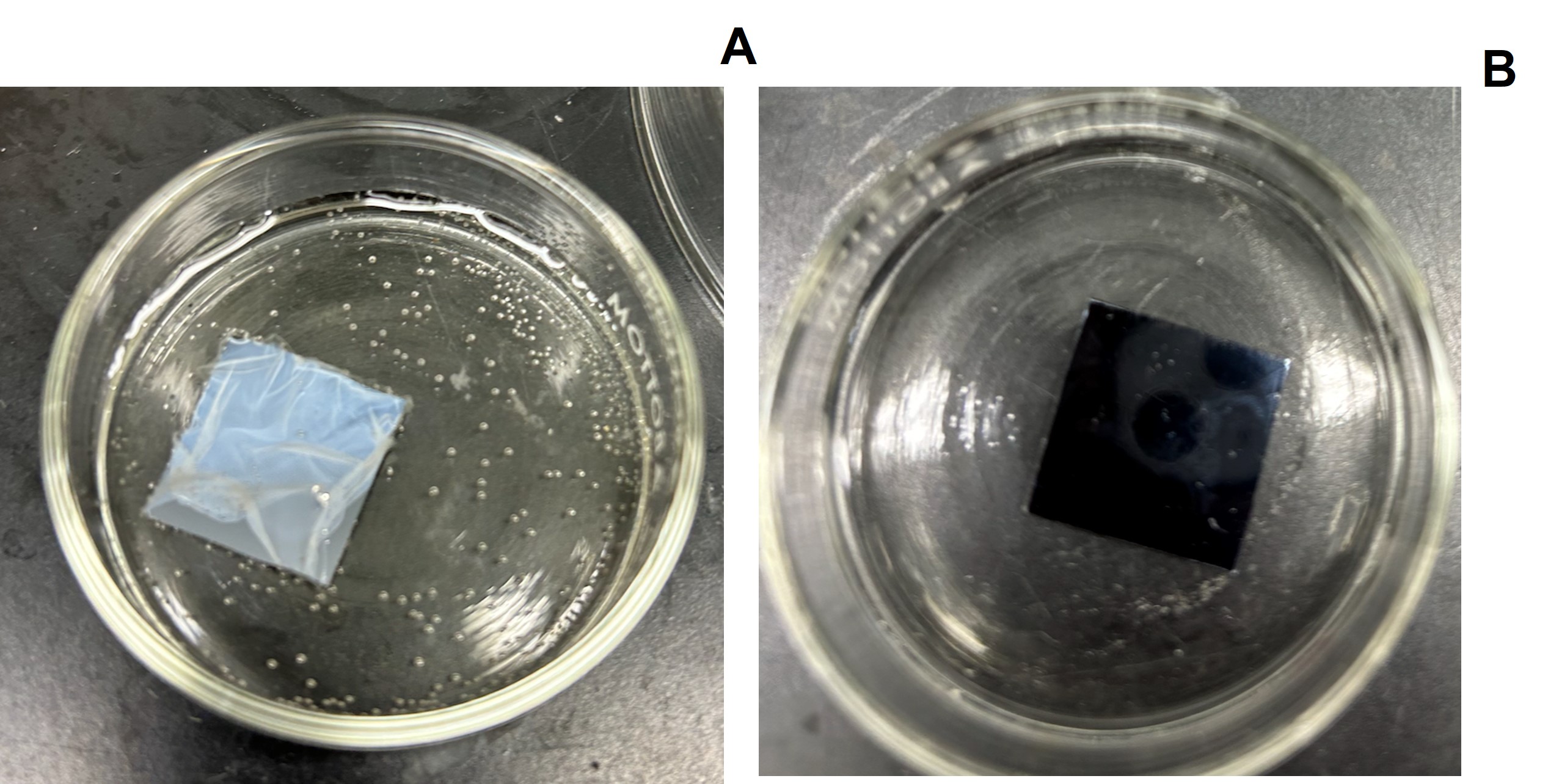
The presence of DCPD in the block-like copolymer massively reduced the degree of swelling that occurred in the membranes and vastly improved stability (Figure 1). The homopolymer is easily withdrawn from the silicon substrate when placed in water, while the copolymer film is firmly attached. This shows that the addition of DCPD had the intended outcome of stabilizing the final copolymer (pDCPD-b-pNBDAC). A lessened degree of swelling will correspond to a consistent value of selectivity.
Surface Characterization Using Contact Angle Goniometry.
The analysis of contact angle goniometry was conducted to assess the surface properties of modified polymer membranes. Analysis showed that both pNBDAC (tris) and pDCPD-b-pNBDAC (tris) had complete wettability, as indicated by a contact angle of <15° (Table 1). A contact angle of <15° is considered completely wet, as it is extremely difficult to measure values lower than this manually. An advancing contact angle of <15° is also consistent with the reported value for hydroxyl groups [10], Additionally, the block-like copolymer appears to completely cover the underling pDCPD, as indicated by the reduction of the contact angle from 86° to <15° (Table 1). This suggests that there is a distinct layer of hydroxyl groups along the surface of the membrane. pNBDAC (taurine) and pDCPD-b-pNBDAC (taurine) did not have massively different contact angles, indicating similar surface properties (Table 2). The contact angle values of pNBDAC (taurine) and pDCPD-b-pNBDAC (taurine) are also consistent with previously reported values of sulfonate modified surfaces [11]. Angle measures are also close to the pDCPD’s contact angle, implying there is not a distinct layer of pNBDAC (taurine) on the top of the membrane. pNBDAC (HA) and pDCPD-b-pNBDAC (HA) had substantially different contact angles. Since the functional group created by the modification of the membranes with hydroxamic acid was expected to be a hydroxyl, a low contact angle would be expected, consistent with the value for pNBDAC (HA). This implies an incomplete layer of hydroxamic acid modified pNBDAC along the surface.
| Table 1. Advancing H2O contact angle measurements (θA) on tris, taurine, and hydroxamic acid modified polymer membranes (pNBDAC, pDCPD-b-pNBDAC) and pDCPD. Table made by student in Microsoft Word. | |
| Membrane | θA (H2O) |
| pDCPD | 86 ± 1° |
| pNBDAC (tris) | <15° |
| pDCPD-b-pNBDAC (tris) | <15° |
| pNBDAC (taurine) | 79 ± 3° |
| pDCPD-b-pNBDAC (taurine) | 75 ± 5° |
| pNBDAC (HA) | 43 ± 12° |
| pDCPD-b-pNBDAC (HA) | 79 ± 4° |
| Table 2. Pervaporation test results for tris, taurine, and hydroxamic acid modified membranes (pNBDAC, pDCPD-b-pNBDAC) and pDCPD. | ||||
| Membrane | Total Flux () | Water Permeance (GPU) | α | % EtOH |
| pDCPD | 1100 | 1800 | 6 | 69 |
| pNBDAC (tris) | 12000 | 24000 | 8 | 64 |
| pDCPD-b-pNBDAC (tris) | 300 | 710 | 10 | 59 |
| pNBDAC (taurine) | 9300 | 5600 | 2 | 85 |
| pDCPD-b-pNBDAC (taurine) | 9200 | 5000 | 1 | 87 |
| pNBDAC (HA) | 3700 | 4000 | 3 | 78 |
| pDCPD-b-pNBDAC (HA) | 9200 | 5000 | 2 | 82 |
Bulk Characterization Using Infrared Spectroscopy.
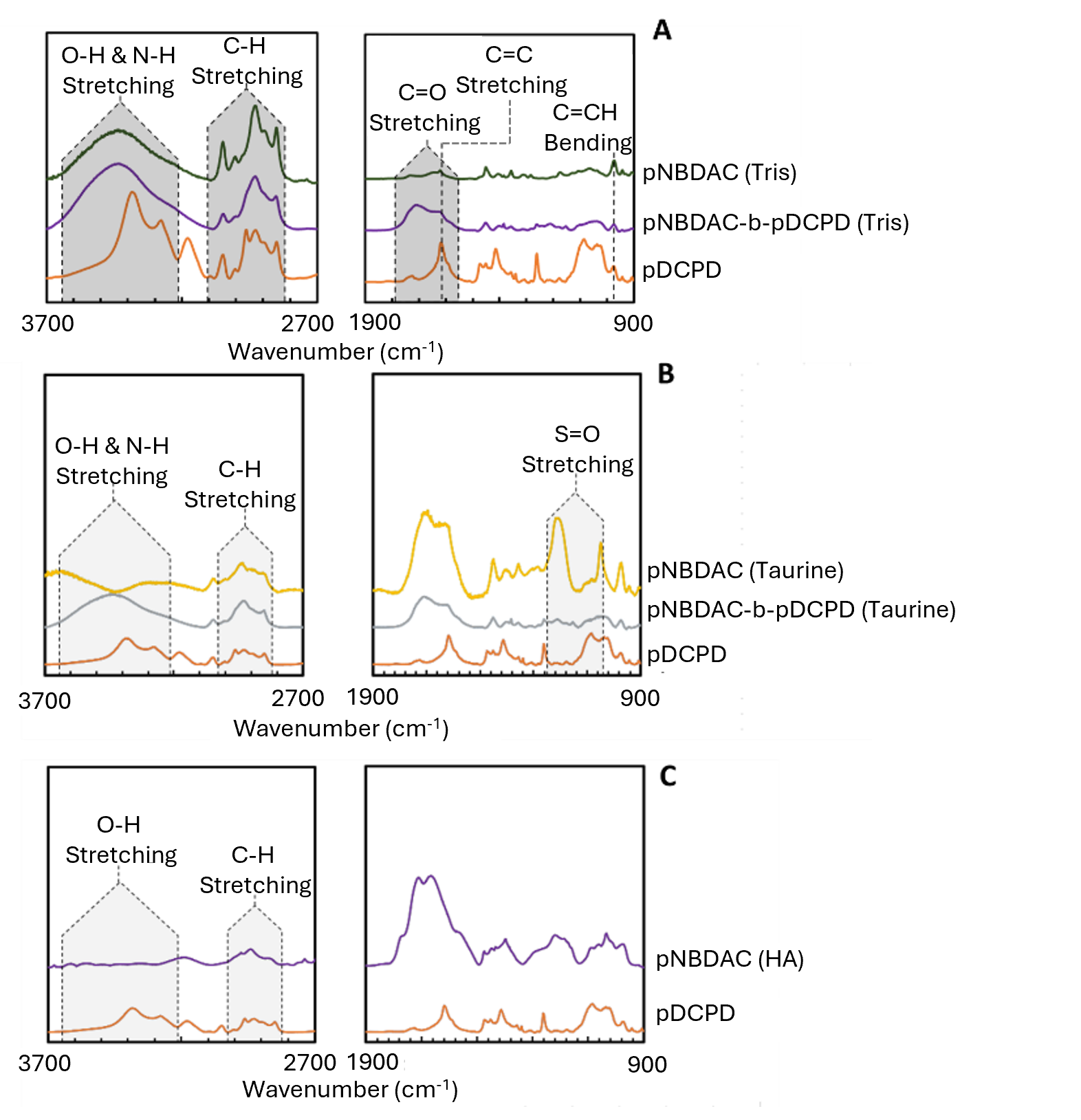
IR spectroscopy was used to determine the bulk composition of the block-like copolymers and confirm synthesis. In the highlighted OH and NH stretching region from 3650 – 3200 cm-1, the pDCPD spectrum has a steep peak that leans towards 3200 cm-1 (Figure 2A). In the pNBDAC (tris) spectrum, the OH and NH stretching region has a wider, more rounded peak. Since this peak is also in the pDCPD-b-pNBDAC (tris) spectrum, it implies the presence of pNBDAC (tris). pDCPD can be seen in the pDCPD-b-pNBDAC (tris) spectrum at the C=C stretching peak at 1550 cm-1. The magnitude of this peak in the pDCPD spectrum is lower than in the pNBDAC (tris) spectrum. In the spectrum of the copolymer, the strength of the C=C peak is similar to the strength of C=C peak in pDCPD, suggesting pDCPD’s presence in the copolymer. Identifiable peaks of both pNBDAC (tris) and pDCPD being present in the spectrum of the copolymer indicate proper synthesis. Figure 2B represents the IR spectra of taurine modified membranes. In these spectra, there is a region of O-H and N-H stretching in which there is a peak that corresponds to the secondary amine present in the modified pNBDAC and pDCPD-b-pNBDAC (taurine). There is also a region of S=O stretching from 1260-1020 cm-1 that corresponds to the sulfonate group. The S=O peak is present in pNBDAC, but not in pDCPD-b-pNBDAC (taurine). This indicates that the block-like copolymer was not fully functionalized. Figure 2C represents the IR spectra of HA modified membranes. The observed C-H stretching peak in the pDCPD and pNBDAC (HA) spectra were expected due to the chemical structure of these polymers. An O-H stretching region was also expected to have a peak in both spectra, but it was flat in the pNBDAC (HA) spectrum. Thus, there is insignificant evidence to imply synthesis of pNBDAC (HA). No spectrum was gathered for pDCPD-b-pNBDAC (HA).
Pervaporation Tests.
Pervaporation tests were conducted to determine the efficiency the synthesized membranes for ethanol dehydration. Total flux corresponds to the amount of mass that is permeating through the membrane’s surface area over time. pNBDAC (tris) had the highest flux (Table 2), which is consistent with its expected high hydrophilicity. The addition of pDCPD into the copolymer appears to have drastically decreased the flux. α represents the selectivity of the membrane. An α >1 means an enrichment of water in the permeate solution. The feed solution enters the system with 90% ethanol and 10% water. Water permeance is the amount of water permeating through the membrane expressed in gas permeance units (GPU). The amount of water permeance is directly correlated to selectivity of the membrane. Overall, the block-like copolymer pDCPD-b-pNBDAC (tris) had the lowest ethanol percentage in the permeate at 59% and a selectivity of 10. PVA has a selectivity of 100 [12], suggesting that further improvements are necessary to make tris modified membranes a viable alternative to distillation. The flux of the two taurine-modified membranes were very similar to each other (Table 2). In terms of selectivity, the value of α for pNBDAC (taurine) and pDCPD-b-pNBDAC (taurine) was lower than even the pDCPD control, which was chosen partly because of its lack of selectivity. Hydroxamic acid modified membranes did not differ in terms of performance, with there being a selectivity difference of 1. Despite the large difference in contact angles, there is a minimal difference in selectivity. Additionally, the higher flux of pDCPD-b-pNBDAC (HA) is consistent with its lower contact angle, as it implies a more hydrophilic surface.
The scROMP technique was successfully utilized to create a block-like copolymer that slightly mimicked the selective properties of PVA. The block-like copolymer that most closely mimicked PVA was pDCPD-b-pNBDAC (tris). This membrane had evidence to suggest proper synthesis with contact angles consistent with those of hydroxyl groups. pDCPD-b-pNBDAC (tris) was also more selective than pNBDAC (tris). This can be attributed to pDCPD reducing the degree of swelling, allowing for the membrane to retain its selectivity. This decrease in swelling could be seen through qualitative observation of membranes. The other block-like copolymers were ineffective at separating water and ethanol. The first hypothesis was refuted, as pDCPD-b-pNBDAC (tris) did not exhibit similar performance to PVA. The selectivity of pDCPD-b-pNBDAC (tris) of 10 was much lower than PVA’s. However, it should be noted that the functional outer block of pDCPD-b-pNBDAC (tris) was only about 100 nm in thickness, whereas PVA membranes are typically much thicker [4]. The second hypothesis was supported, as the homopolymer pNBDAC (tris) exhibited a large degree of swelling, with large wrinkles and most of the membrane withdrawn from the substrate. pDCPD-b-pNBDAC (tris) – a block-like copolymer containing DCPD – remained entirely adhered to the surface. This indicates a lower degree of swelling.
CONCLUSION.
This study sought to create a block-like copolymer for ethanol dehydration that exhibited the functionality of PVA while still allowing for rapid synthesis via scROMP. This study also sought to minimize swelling in our hydrophilic membrane. To do this, a block-like copolymer was synthesized using pNBDAC and pDCPD. The created copolymer was then modified in one of three modifying solutions (tris, taurine, hydroxamic acid). After this, membrane performance was evaluated using pervaporation tests. The membranes were also characterized to confirm proper synthesis. The results showed that the three synthesized block-like copolymers were much less selective than PVA but were still more selective than the unmodified homopolymer films of either block while exhibiting less swelling. Characterization implied that some membranes were not fully functionalized. Overall, these results show that block-like copolymers do increase the selectivity of some monomers. This study also shows that block-like copolymers in modifying solutions could provide an effective alternative to high-energy chemical separation processes such as distillation.
ACKNOWLEDGMENTS.
The author would like to acknowledge the Jennings Group at Vanderbilt, the Vanderbilt Collaborative for STEM Education and Outreach, and the Interdisciplinary Science and Research program at John Overton High School.
REFERENCES.
- V. Delgado Bastos, “Etanol, alcoolquímica e biorrefinarias” (Banco Nacional de Desenvolvimento Econômico e Social, 2007); https://web.bndes.gov.br/bib/jspui/bitstream/1408/2527/1/BS%2025%20Etanol%2c%20Alcoolqu%c3%admica%20e%20Biorrefinarias_P.pdf
- D. S. Sholl, R. P. Lively, Seven chemical separations to change the world. Nature 532, 435–437 (2016)
- USDA Releases New Report on Lifecycle Greenhouse Gas Balance of Ethanol (USDA, 2017); https://www.usda.gov/media/press-releases/2017/01/12/usda-releases-new-report-lifecycle-greenhouse-gas-balance-ethanol.
- K. S. Burts, T. V. Plisko, A. V. Bildyukevich, G. Li, J. Kujawa, W. Kujawski, Development of dynamic PVA/PAN membranes for pervaporation: Correlation between kinetics of gel layer formation, preparation conditions, and separation performance. Chemical Engineering Research and Design 182, 544–557 (2022).
- M. Dmitrenko, A. Zolotarev, T. Plisko, K. Burts, V. Liamin, A. Bildyukevich, S. Ermakov, A. Penkova, Effect of the Formation of Ultrathin Selective Layers on the Structure and Performance of Thin-Film Composite Chitosan/PAN Membranes for Pervaporation Dehydration. Membranes 10, 153 (2020).
- Z. J. Parkerson, L. Prozorovska, M. P. Vasuta, T. D. Oddo, G. K. Jennings, Simultaneous Spin Coating and Ring-Opening Metathesis Polymerization for the Rapid Synthesis of Polymer Films. ACS Appl. Mater. Interfaces 16, 16754–16766 (2024).
- G. K. Jennings, E. L. Brantley, Physicochemical Properties of Surface‐Initiated Polymer Films in the Modification and Processing of Materials. Advanced Materials 16, 1983–1994 (2004).
- J. A. Love, J. P. Morgan, T. M. Trnka, R. H. Grubbs, A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile. Angew. Chem. Int. Ed. 41, 4035–4037 (2002).
- X. Deng, L. Prozorovska, G. K. Jennings, Metal Chelating Polymer Thin Films by Surface-Initiated ROMP and Modification. J. Phys. Chem. C 123, 23511–23519 (2019).
- F. D. Petke, B. R. Ray, Temperature dependence of contact angles of liquids on polymeric solids. Journal of Colloid and Interface Science 31, 216–227 (1969).
- L. Bao, Y. Yang, J. Li, X. Li, J. Dong, Synthesis and structure–property relationship of coal-based isomeric alkylbenzene sulfonate surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 696, 134350 (2024).
- D. Van Baelen, B. Van Der Bruggen, K. Van Den Dungen, J. Degreve, C. Vandecasteele, Pervaporation of water–alcohol mixtures and acetic acid–water mixtures. Chemical Engineering Science 60, 1583–1590 (2005).
Posted by buchanle on Wednesday, June 25, 2025 in May 2025.
Tags: Copolymer, ethanol, membrane, pervaporation, separation

