Innovating Green Energy Generation: On the Development of a Filter for Fossil Fuel Power Plants’ Chimneys to Minimize Emis-sions of Exhaust Gases and Produce Methanol
ABSTRACT
In the current era of the increasingly intense concomitants of the climate crisis, the high concentration of toxic substances and greenhouse gases in the chimneys of fossil fuel power plants plays a major role in the continuation of events like the ozone hole, acid rain, the greenhouse effect, and the degradation of biotopes. The objective of this paper is to theoretically develop a filter to be placed in the chimneys of such plants to minimize the environmental footprint of their gaseous pollutants and to produce useful side-products, such as methanol and SO42-/NO3– anions. Should this study be practically effective, it will have multiple benefits, since methanol has a wide range of applications in the chemical and energy industries [1], and the sulfate and nitrate ions are widely utilized in fertilizers.
INTRODUCTION.
The industrial sector and fossil fuel power plants have emitted 37.15 billion metric tons of carbon dioxide (CO2) in 2022 alone [2] (which makes up roughly 76% percent of all greenhouse gas emissions), along with 28.5 and 201.6 million metric tons of nitric oxides (NOX) and sulfur dioxide (SO2), respectively. Past bibliographical attempts to address this issue have focused on the removal and storage solely of emitted CO2 from fossil fuel power plants, or on its separation from the other released gases, or on its utilization to produce methanol [3], but no mechanism combining these techniques has yet been published. Thus, this proposed filter aims to both decrease the emissions of all CO2, SO2, and NOX gases and to use them to form useful side-products. This arrangement will be placed in the chimneys of the plants and operate as follows:
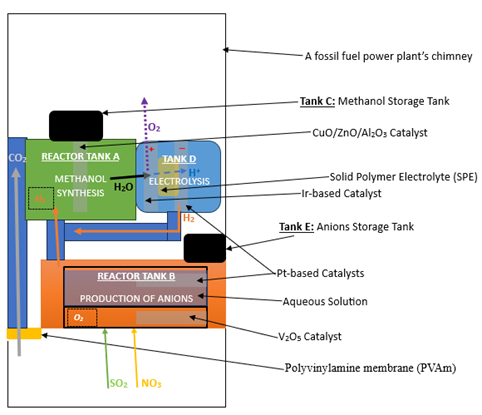
1) The gases in the chimney will be separated via a polymeric membrane, with CO2 being channeled into Reactor Tank A and SO2 and NO channeled into Reactor Tank B.
2) In Tank A, CO2 reacts with H2 to produce methanol, which is purified and stored in Tank C.
3) The H2O produced in Tank A will be moved into Tank D, where it will be electrolyzed to produce H2 and O2. The H2 produced will then be channeled into Tank A as a reactant in the methanol synthesis reaction.
4) In Reactor Tank B, SO2 and NO will dissolve in H2O and become acidified and then ionized. The H+ cations will become H2 and move into Tank A. The remaining SO42- and NO3– anions will then be absorbed from water and stored in a separate Tank E.
These steps provide a brief overview of the processes involved in the filter. In the next sections of this paper, the practical methods that will be utilized for the realization of these processes shall be discussed, based on the published literature of the field and previous experimental results (Fig. 1).
MATERIALS AND METHODS.
Emission Rates.
The 2022 CO2, SO2, and NOx emission data of all US fossil fuel (electricity-generating) power plants have been used for this estimation [4], as not only are they the most up-to-date emission figures available, but also because 2022 was the year with the most CO2 emissions in the entire human history. By calculating the mean values of the available data and considering that NO accounts for anywhere between 95-99% of the total NOx in the gas stream [5], it can be concluded that 9.889kg of CO2, 5.676g of SO2, and 6.1453g of NO are emitted per second by a fossil fuel power plant. All other substances in the exhaust gases are considered negligible due to their comparatively low amounts.
CO2 Capture.
The separation of CO2 from the other exhaust gases will be achieved through a Carbon Capture and Storage (CCS) technique and more particularly a polymeric membrane. In comparison to other technologies (absorption, chemical looping, etc.), a polymeric membrane operates at lower temperatures and pressures (lower energy requirements); allows high selectivity of CO2 over the other gases; and can be scaled easily, allowing for adjustments based on the CO2 capture needs of a facility, ranging from pilot plants to large industrial applications. To this end, the membrane should have high permeability and selectivity (great thinness) and be physically, chemically, and thermally stable. Thus, no inorganic materials will be used, because of their poor reproductivity, brittleness, and high cost; contrariwise, the high area-to-volume ratio, low cost, and good processibility of organic membranes make them ideal for this purpose [6]. Indeed, Polyvinylamine (PVAm) will be used, as it provides a 90% post-combustion purity and recovery rate for carbon dioxide. It also has good stability for over 6.5 months and a CO2/N2 selectivity of 300, resulting in minimum SO2 and NOx impurities because of the high enough selectivity of carbon dioxide over sulfur dioxide and nitrate oxides [7]. Recently, it has been redeveloped from a flat sheet into a hollow fiber configuration with a membrane area of 4-18 m2 [8].
The gas will be transported via a solution-diffusion (SD) carbon dioxide membrane-separation process, which shall encompass the following three steps: firstly, absorption; next, dissolution and diffusion; and, finally, desorption. Initially, as the exhaust gases rise in the power plant’s chimney, they all come into contact with the surface of the membrane; due to the driving force of a partial pressure difference (higher CO2 concentration on the feed side compared to the permeate side) and the high selectivity and permeability of PVAm for CO2 relative to SO2 and NOx, carbon dioxide molecules are attracted to and adsorbed onto the membrane surface. Afterward, CO2 dissolves into the membrane material, penetrates into its bulk, and diffuses through its matrix. Lastly, the carbon dioxide molecules, now surrounded by the membrane’s polymeric structure, reach the permeate side of the material, where the concentration of CO2 is lower due to the continuous removal of gas, creating a driving force for it to exit the membrane. The gas molecules desorb from the membrane surface into the permeate side, effectively separating them from other gases in the feed stream [9]. As the average efficiency of the CCS processes is 90%, 8.9kg of CO2 will be ultimately channeled into Tank A per second.
Methanol Synthesis.
The first step of the filter mechanism involves the process of methanol synthesis. Industrially, CH3OH is produced through the hydrogenation of carbon monoxide (CO) and carbon dioxide (CO2) over an active catalyst under high pressure (Reactions 1-3).
CO2(g) + 3H2(g) ⇌ CH3OH(g) + H2O(g) [CO2 Hydrogenation] (1)
CO(g) + 2H2(g) ⇌ CH3OH(g) [CO Hydrogenation] (2)
CO2(g) + H2(g) ⇌ CO(g) + H2O(g) [Water gas shift reaction] (3)
Reactions (1) and (2) are exothermic and reversible, so noteworthy cooling is required to expel excess reaction heat to shift equilibrium towards the products and form more methanol. However, the kinetics of the reactions are not favored at lower temperatures, so an optimum temperature (250-300°C), higher pressure (50-100 bar), and active Cu- based catalysts have to be used to obtain high conversions of syngas to methanol [10]. More particularly, conventional over copper-based catalysts CuO/ZnO/Al2O3 (at percentages 60-70%, 20-30%, and 5-15% respectively) [11] should be preferred on this occasion, since Copper (Cu), an extremely selective catalyst, converts 99.5% of CO and CO2 into CH3OH in gas-solid catalytic reactors [1]. After all, even in conditions of lower (atmospheric) pressure (and high temperature), this mixture is widely accepted as the best methanol synthesis catalysts [12]. As far as their efficiency is concerned, when 5% palladium was also added, the palladium-based Pd/Cu/Zn/Al2O3 catalyst had a selectivity of 64% at 210 °C and 5 bar [13]. Although the conditions apparent in our filter (significantly higher pressure and temperature) will lead to a substantially higher equilibrium yield, it will be assumed that this process yields methanol and water at a 64% level, so as to assess the baseline efficiency of this model.
As has been mentioned, 8,900 gs-1 CO2 are absorbed by the CCS polymeric membrane and will thus react in Tank A with H2 molecules. Nonetheless, reactions (1) and (3) show that part of CO2 will react with H2 to produce methanol and water, and part of CO2 will react with H2 to form CO, which will in turn react with H2 to form methanol (2). The bibliographical literature shows that the conversion rates of reactions (1) and (3) are approximately 70%-90% and 10%-30%, respectively, for the stoichiometric ratio to hold true [14] (Table 1).
| Table 1. Stoichiometric Calculations for Methanol Synthesis | ||
| Substance | Initial Amount | Final Amount |
| Carbon Dioxide CO2 | 202.308 mol | 0 mol (fully consumed) |
| Carbon Monoxide CO | 0 mol | 0 mol (fully consumed) |
| Hydrogen H2 | 606.924 mol | 0 mol (fully consumed) |
| Methanol CH3OH | 0 mol | 129.477 mol |
| Water H2O | 0 mol | 129.477 mol |
After all reactions of the process are completed, the products are a gaseous mixture of methanol CH3OH (g) and water H2O (g). To separate them, a thermally efficient two-column distillation system for enhanced methanol purification, optimized energy consumption, and reduced heat input will be used. In the first column, the crude methanol-water mixture undergoes distillation, forming a methanol product stream, a water-rich bottoms system, and an aqueous methanol side stream (containing at least 95% methanol). The latter is then sent to a second column where further separation occurs, yielding a purified methanol product stream and a bottoms liquid with less than 60% water. In both cases, distillation is achieved based on the different boiling points of methanol and water, which lie at 64.7°C and 100°C, respectively. This configuration allows for efficient use of heat, as the reboilers of the columns can be heated by low-pressure steam or process gases, utilizing heat recovery from the methanol synthesis and synthesis gas generation steps. Also, this model implements further sustainability practices beneficial to large-scale methanol production processes, such as recycling methanol vapors for heat exchange and using low-grade heat sources like process gas or low-pressure steam (which are commonly available in methanol production plants) [15]. Thereby, methanol will move upwards into Tank C, while the recovered 40% of water (51.7908 mol) will be channeled into Tank D, where it will be electrolyzed to produce hydrogen molecules (H2). Additionally, a very small amount of it (0.156867 moles) will be channeled into Tank B, so that the other gases emitted by the power plant can be dissolved in it. Given the efficiency of methanol distillation at 95%, 123.003 mol CH3OH will be finally produced during the distillation process.
Water Electrolysis.
Albeit the production of methanol in the Reactor Tank A is considerably significant for the minimization of CO2 emissions, it is also bound to multiple costs, since it requires considerable amounts of energy (it will be performed in an environment of 250-300°C, and even for its purification much heat must be provided) and a high amount of H2 molecules (606.924 mol/s). Thus, upholding the principles of green chemistry, the 51.552933 moles of water produced from the methanol synthesis must not be regarded as waste, but instead as a useful source of hydrogen molecules (H2) production, which shall be once again provided to Reactor Tank A for methanol to be synthesized. Herein, this reaction’s technical parameters and chemical background will be analyzed.
In particular, Proton Exchange Membrane (PEM) Electrolysis, which uses a Solid Polymer Electrolyte (SPE) to conduct protons from the anode to the cathode while insulating the electrodes electrically, will be utilized in Tank D. Apart from the conduction of protons, the SPE is also responsible for the separation of product gases and the electrical insulation of the electrodes. The mechanics behind this procedure is that in the anode, the H2O is converted into oxygen molecules (O2) and hydrogen cations (H+); the H+, contrary to the O2, can be then transferred through the membrane to the cathode, while the oxygen is released. Afterward, the H+ cations become hydrogen molecules (H2) and are channeled from Tank D to Tank A. The relevant equations are provided below: (4) and (5) determine the half ionic equations for oxidation and reduction, respectively, while (6) represents the full ionic equation.
Anode – Oxygen Evolution Reaction (OER):
2H2O(g) → O2(g) + 4H+(aq) + 4e– (4)
Cathode – Hydrogen Evolution Reaction (HER)
4H+(aq) + 4e– → 2H2(g) (5)
System – Full Ionic Equation
2H2O(g) → O2(g) + 2H2(g) (6)
The PEM approach was selected because of its numerous benefits, encompassing its commercial applicability, easy maintenance, remarkably high product gas purity (because of its low gas crossover rate) [16], great efficiency, low gas permeability, extremely thin membrane, and ability to function at high current densities [17], high pressures and a wide range of temperatures, leading to minimal ohmic and energy losses.
The process operates at temperatures between 20-80°C with a membrane length of 100-200μm and current density of 1-3A/cm². Theoretical electrical potential differences for the electrolyzer range from 1.23 to 1.48V, but practical applications typically require 1.8-2.0V due to overpotentials at the electrodes. Energy consumption is estimated at 4.0-5.0kWhN-1m-3, with an assumed efficiency of about 80% [18]. The cathode utilizes platinum-based materials as electrocatalysts for the HER, while iridium-based catalysts are preferred for the OER at the anode, owing to their stability and performance [19]. Nafion membranes are favored for their high proton conductivity and mechanical strength [20]. The electrolyzer is designed for a lifespan of 80,000 hours, maintaining a minimum hydrogen/oxygen safety ratio of 4 mol% in the oxygen stream, as is ensured by the selected membrane’s quality.
Since the efficiency of PEM electrolysis is approximately 80%, 41.2423464 mol H2 will be finally produced per reaction; 565.682 moles H2 still must be provided for each CH3OH synthesis.
Anion Production.
The final chemical process of this model encompasses the production of SO42- and NO3– anions by sulfur dioxide (SO2) and nitrogen monoxide (NO); as SO2 and NO rise into Tank B, after being separated from CO2, they will initially mix with a gas stream of oxygen molecules (O2), then be dissolved in an aqueous solution (H2O), and finally react with a Pt-based metal catalyst to perform a HER.
2SO2(g) + O2(g) ⇌ 2SO3(g) (7)
SO2(g) + V2O5(s) → SO3(g) + 2VO2(g) (8)
2VO2(g) + 3O2(g) → 2V2O5(s) (9)
2NO(g) + O2(g) → 2NO2(g) (10)
Reactions (7)-(9) represent the oxidation of sulfur dioxide. Vanadium pentoxide (V2O5) will be used as a catalyst to amplify the interactions between the gases by oxidizing the SO2 to SO3 (7) and then re-oxidizing itself (8, 9) with the aid of the oxygen gas stream. Its reduction to vanadium oxide (VO2) ensures that a catalytic circle will be followed. According to Le Chatelier’s Principle, the gas temperature must be maintained at least at 400°C to achieve a near 100% conversion rate of SO2 into SO3 [21]; after all, below this temperature, V2O5 is inactive as a catalyst, and above 620°C, it breaks down. Similarly, nitrogen monoxide can become oxidized under these parameters as well (10), with V2O5 catalytically enhancing its conversion into NO2. During this process, all gases should pass through a four-stage reaction vessel, with each stage consisting of a solid catalyst bed.
SO3(g) + H2O(l) → H2SO4(aq) (11)
3NO2(g) + H2O(l) → 2HNO3(aq) + NO(aq) (12)
The second step is the dissolution of the produced SO3 and NO2 in pure water, H2O. For (11) and (12) to be effectively carried out, the gases must be moved into an absorption tower, where more than 98.5% of SO3(g) will be converted to H2SO4(aq) at 70°C. Given that sulfur trioxide reacts violently with water, a fog of concentrated sulfuric acid droplets will be produced, dissolved in an aqueous solution. At such temperatures, nitrogen dioxide is soluble enough to become nitric acid (dissolved in the aqueous solution), when it comes in contact with water.
H2SO4(aq) → 2H+(aq) + SO42-(aq) (13)
HNO3(aq) → H+(aq) + NO3–(aq) (14)
However, both H2SO4(aq) and HNO3(aq) are strong acids and thus will become completely ionized in the aqueous solution, dissociating into their respective ions, H+, SO42-, and NO3–, without any further addition of water (13, 14).
The third step of this process is the inclusion of Pt-based metal catalysts in the reaction mixture, which reduces all H+ cations into H2 molecules, in a similar fashion to the cathode side of the PEM electrolysis (5). Since this process holds only true for T≤80°C but takes place at T=70°C, it can be realized without additional chemical requirements. The reaction mixture contains now only SO42-(aq) and NO3–(aq) anions, together with the toxic NO(aq), which, albeit not further providing any useful anions, has not been released in the atmosphere; given its high toxicity, this enhances even more the environmental significance of the filter at hand.
Given the existing technical conditions, as described in the section above, the stoichiometry of all (5), (7)-(14) reactions will be calculated at their given yield of 98.5% (Table 2).
| Table 2. Stoichiometric Calculations for Anion Production | ||
| Substance | Initial Amount | Final Amount |
| Sulfur Dioxide SO2 | 0.0886 mol | 0 mol (fully consumed) |
| Nitrogen Monoxide NO | 0.2048 mol | 0.068267 mol (dissolved in the reaction mixture; not released into the atmosphere) |
| Water H2O | 0.156867 mol | 0 mol (fully consumed) |
| SO42- anions | 0 mol | 0.08417 mol |
| NO3- anions | 0 mol | 0.12970 mol |
| Hydrogen H2 | 0 mol | 1.19212 mol |
Albeit the total amount of H2 produced from this procedure is small compared to the overall H2 requirements of the methanol synthesis process, ultimately 564.4895 moles of H2 must still be provided for CH3OH synthesis per second.
Economic Considerations.
After the chemical processes followed in the proposed filter have been described, its economic feasibility should be considered in terms of its energy consumption/function costs and the respective earnings from the produced methanol. Since a fossil fuel power plant chimney normally works at an average temperature of 150°C and pressure of 0.1 bar, a total of 7.4675 MJ/s are required to maintain the necessary conditions (250°C and 50 bar) for the methanol synthesis to occur at the desired rate, according to the equation of calorimetry and an adiabatic-isothermal process approximation, given that the isobaric specific heat capacity of carbon dioxide is 28.96 kJkg-1K-1. The energy consumption for the third stage of the model is negligible, as the reactions occur at the already existing conditions of the chimney. Nevertheless, the main energy consumption cost is the necessary electricity for the water electrolysis to take place, which amounts to 285.83 kJ/mol, or, more specifically, 14.73537484 MJ/s. Taking into account that 0.1626 USD corresponds to 1kWh (or 3.6MJ) of energy, the total energy-related costs amount up to 1.002829847 USD/s. Additionally, the cost of the utilized H2 amounts to 0.6308383414 USD/s since 1.80 kg of H2 cost 1 USD on average. Conversely, 1.70251896384 USD are earned per second due to the production of methanol, whose commercial value is 432 USD per metric ton. Therefore, 0.06885077544 USD/s are earned as a result of this model’s utilization, hence proving its profitability for power plants. For these calculations, publicly available values from the scientific literature and commercial industries have been utilized. Additional factors that could have been considered but are difficult to estimate on a general theoretical framework include the cost per second for CO2 capture and the respective earnings from turning the produced anions into material for fertilizers.
DISCUSSION.
CO2, SO2, and NO emissions are cornerstones of the climate crisis’s perpetuation. Therefore, the proposed filter aims to minimize the releases of such dangerous gases, so as to limit the environmental footprint brought about by the function of fossil fuel power plants, while also making this technology profitable to such industries through the production of methanol and other substances essential for fertilizers. Moreover, this paper delves into the technical aspects of this mechanism, determining the conditions of temperature and pressure that must exist for more than one reaction to effectively occur in each tank per second and providing a framework applicable to the majority of fossil fuel power plants, despite their potential minor in-between differences. Finally, not only does this filter build upon past credible chemical protocols, but it also favors the principles of green chemistry, as it recycles reaction by-products (such as H2O) and uses them to minimize operation costs.
Future research in this area should encompass the development of a functional model based on this theoretical approach, in order to practically assess the viability and efficiency of the idea at hand and measure any potential drawbacks that could not be estimated through a sole theoretical analysis. To amplify the financial benefits of this model, approximately 4,075 solar panels could be placed on each power plant, thus minimizing the energy consumption costs of the model and promoting the usage of alternative energy sources. Green chemistry is the future in many ways, engineering solutions that enhance the currently existing technology, providing a sustainable framework for society to build upon and minimizing the human impact on the planet’s natural processes. Thus, as far as the power plants are concerned, at least, carbon capturing through a chimney filter is the path to follow.
SUPPORTING INFORMATION.
Supporting Information includes detailed stoichiometric and financial calculations for each chemical process step of the proposed model, supporting the final results listed in this paper.
REFERENCES.
- B. Balopi, P. Agachi, Danha, Methanol Synthesis Chemistry and Process Engineering Aspects – A Review with Consequence to Botswana Chemical Industries, Procedia Manufacturing, 35, 367-376 (2019).
- H. Ritchie, M. Roser, CO₂ emissions, Our World in Data (2020); https://ourworldindata.org/co2-emissions
- S. Nagireddi, J.R. Agarwal, D. Vedapuri, Carbon Dioxide Capture, Utilization, and Sequestration: Current Status, Challenges, and Future Prospects for Global Decarbonization, ACS Engineering AU. 4(1), 22-48 (2023).
- Emissions by plant and by region, U.S. Energy Information Administration, (2022); https://www.eia.gov/electricity/data/emissions/
- P. Zhang, Y. Shao, J. Niu, X. Zeng, X. Zheng, C. Wu, Effect of low-nitrogen combustion system with flue gas circulation technology on the performance of NOx emission in waste-to-energy power plant, Chemical Engineering and Processing – Process Intensification, 175, 108910 (2022).
- L.M. Robeson, The upper bound revisited, Journal of Membrane Science. 320, 390–400 (2008).
- W. Yave, A. Car, S.S. Funari, S.P. Nunes, K.-V. Peinemann, CO2-philic polymer membrane with extremely high separation performance, Macromolecules. 43, 326–333 (2009).
- B. Xue, X. Li, L. Gao, M. Gao, Y. Wang, L. Jiang, CO2-selective free-standing membrane by self-assembly of a UV-crosslinkable diblock copolymer, Journal of Materials Chemistry. 22, 10918– 10923 (2012).
- Y. Han, W.S. Winston Ho, Polymeric membranes for CO2 separation and capture, Journal of Membrane Science. 628, 119-244 (2021).
- O. Mäyrä, K. Leiviskä, Modeling in Methanol Synthesis, Methanol, 475–492 (2018).
- H. Bakhtiary, F. Hayer, ‘‘Kinetics and Reactor Modeling of Methanol Synthesis from Synthesis Gas’’ in European COMSOL Multiphysics Conference, Hanover (2008).
- I. Sharafutdinov, “Investigations into low pressure methanol synthesis, Sharafutdinov,” thesis, Department of Physics, Technical University of Denmark (2013).
- Trifan, B., Lasobras, J., Soler, J., Herguido, J. & Menéndez, M. Modifications in the composition of CuO/ZnO/Al2O3 catalyst for the synthesis of methanol by CO2 hydrogenation. Catalysts, 11(7), 774 (2021).
- A.M. Ribeiro, J.C. Santos, A.E. Rodrigues, S. Rifflart, Syngas Stoichiometric Adjustment for Methanol Production and Co-Capture of Carbon Dioxide by Pressure Swing Adsorption, Separation Science and Technology. 47(6), 850–866 (2012).
- A. Pinto, “Methanol distillation process,” (US Patent 4210495A, March 11, 1977). [US4210495A]
- M. Carmo, D. Fritz, J. Mergel, D. Stolten, A comprehensive review on PEM water electrolysis, International Journal of Hydrogen Energy. 38 (12), 4901–4934 (2013).
- S. Slade, S.A. Campbell, T.R. Ralph, F.C. Walsh FC, Ionic conductivity of an extruded Nafion 1100 EW series of membranes, Journal of the Electrochemical Society. 149(12), A1556 (2002).
- Q. Lu, Y. Yu, Q. Ma, B. Chen, H. Zhang, 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions, Advanced Materials. 28, 1917–1933 (2016).
- T. Wang, X. Cao, L. Jiao, PEM water electrolysis for hydrogen production: fundamentals, advances, and prospects, Carb Neutrality. 1(21), (2022).
- C. Immerz, M. Paidar, G. Papakonstantinou, B. Bensmann, T. Bystron, T. Vidakovic-Koch, K. Bouzek, K. Sundmacher, R. Hanke-Rauschenbach, Effect of the MEA design on the performance of PEMWE single cells with different sizes, Journal of Applied Electrochemistry. 48, 701–711 (2018).
- J. Simpson, J. Petherick, L. Donaldson, The Manufacture of Sulfuric Acid and Superphosphate, Chemical Processes in New Zealand, (2018).
Posted by buchanle on Friday, June 20, 2025 in May 2025.
Tags: carbon capture, fossil fuel power plant, green chemistry, methanol synthesis

