Sleep Deprivation and a High Fat Diet During Adolescence Does Not Affect Hippocampal Structure in Adulthood
ABSTRACT
It is well documented that sleep deprivation and a high fat diet during adolescence hurts hippocampal structure, but it is unknown whether these deficits persist into adulthood. In this study, we used cresyl violet and doublecortin stains to measure hippocampal volume and neurogenesis, respectively, in adult rats who were sleep deprived and given a high fat diet during adolescence. We found that there was no significant difference in either hippocampal volume or neurogenesis compared to adult rats given no manipulation during adolescence or given just a high fat diet. While decreased hippocampal volume and neurogenesis both cause mental health issues such as depression and stress, our results suggest that these negative conditions during adolescence can be reversed in adulthood.
INTRODUCTION.
Studies have revealed that adolescence is a critical period for brain development [1]. However, many teenagers engage in poor eating habits and lack the necessary amount of sleep. Children aged 11-17 years old are recommended to get 8 to 10 hours of sleep a night, but 45% of teens get less than 8 hours of sleep on weekdays and 43% get less than 8 hours on weekends [2]. Sleep deprivation increases the likelihood of obesity and can result in people making poor diet choices [2,3]. These behaviors individually are shown to increase stress and are related to depression and memory loss [2,4]. However, the effects of a combination of sleep deprivation and a high fat diet into adulthood have not been investigated thoroughly. Since a trial on humans would take too long and be difficult to experimentally control, a rodent model is more suitable. Rodents are comparable to humans since they are behaviorally and biologically similar, so any experimental results could translate.
Deficits in hippocampal volume and neurogenesis, the formation of new neurons, are both indicators of stress, depression and memory loss [5]. Studies have shown that rats given a high fat diet exhibit lower hippocampal volume and levels of neurogenesis during adolescence, but if they were taken off this diet after adolescence into adulthood, there was less of a decrease [6]. The effects were also less severe if the rats were given a high fat diet only during adulthood [5]. Moreover, rodents that were sleep deprived were shown to have lower levels of neurogenesis and lower hippocampal volume [7]. It is unknown, however, whether the combination of the two will have long term effects on brain structure in adulthood. Therefore, the purpose of this research is to determine if rats that were sleep deprived and given a high fat diet during adolescence will have lower hippocampal volume and levels of neurogenesis in adulthood.
MATERIALS AND METHODS.
Animals and Experimental Methods.
Twenty-three-week-old Long-Evans rats, a breed commonly used in labs, were housed in groups of 3-4 for one week to acclimate to their surroundings and were given a normal 12-hour light/dark cycle to sleep before being randomly assigned into either a control group (n=6) given a diet with 13% of calories coming from fat, a group given the high-fat diet (HFD) (n=6) given a diet with 45% of calories coming from fat, or a group given the HFD and deprived of sleep (n=7). Both diets used the same types of fat and rats were weighed to make sure there was a weight difference between the HFD groups and the control. The control and HFD groups were given a normal 12-hour light and dark cycle. The HFD and sleep deprivation group was given the high fat diet and a 24-hour dark cycle in a light deprivation box. After the manipulation period when the rats are 8 weeks old, all rats return to a normal diet and sleep schedule for 7 more weeks.
Brain Processing.
Once the rats reached 15 weeks old, they were euthanized via rapid decapitation. Their brains were extracted and stored in 4% paraformaldehyde for over 24 hours. Then the brains were transferred into 40% sucrose and stored for over 48 hours. One hemisphere from each brain was frozen with dry ice and cut into 40 μm thick slices using a microtome. The slices were stored in 1 N phosphate buffered saline (PBS) solution.
Cresyl Violet Stain.
Slices from each brain were mounted onto slides and were allowed to dry. The dried slices were dyed in a 0.1% cresyl violet solution to stain for 4 to 6 minutes. Then each slide was quickly dipped in distilled water and then placed in 100% ethanol, 95% ethanol, and 70% ethanol each for 3 minutes to rinse off the excess dye. Afterwards, each slide sat in distilled water for 5 minutes and finally xylene to clear the slices for another 5 minutes. The hippocampus appears dark purple in the stained slices due to the higher density of neurons in this region of the brain. Thus, the area of the hippocampus can be measured for each slice under a normal light microscope.
Doublecortin Stain.
Slices from each brain were moved from the wells of PBS and rinsed three times for 5 minutes in PBS before being placed in a blocking solution made of 5% tween-20 and normal donkey serum for five minutes to prepare the tissue for the antibody. Afterwards the tissue was placed in a solution with the rabbit antibody, which is needed for the fluorescent antibody to bind to the new neurons, and stored for 72 hours. The tissue was removed from the fridge and rinsed three times with PBS for five minutes. Then it was placed in a solution with rabbit-anti Alexa fluorescent antibody, which makes the neurons visible, and stored in the dark for 1 hour. The tissue was taken out and rinsed three times in PBS for five minutes before being placed in Hoechst for five minutes which confirms if the stain works if no cells are visible and rinsed again in PBS three times. The tissue was mounted on slides and cover slipped with glycerol. Then the tagged neurons were counted for each slide under a mercury-bulb fluorescent microscope and the total amount of new neurons per slice was approximated for each brain.
RESULTS.
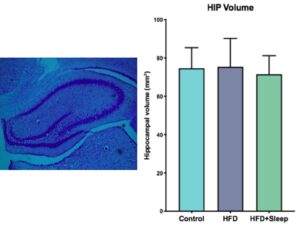
We estimated hippocampal volume by measuring the area of the hippocampus in each slice and summing over the total number of slices for each brain (Figure 1a). We took the average volume of each manipulation group and performed a one-way ANOVA to determine significance, which determined there was no significant difference between the groups (p=0.817, p>0.05) The differences in hippocampal volume are shown in Figure 1b.
Figure 1. Cresyl Violet Stain Data a) Image of the cresyl violet stain with the hippocampus outlined by the band of dark purple stained cells b) The results from measuring hippocampal volume indicate there is no significant difference between the groups. The error bars represent one SEM.
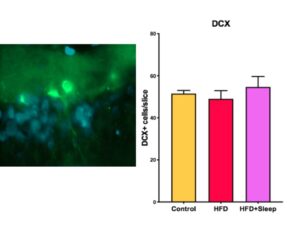
We quantified neurogenesis by counting the number of doublecortin tagged cells in the slice (Figure 2a). Then we took the average number of neuronal cells per slice for each group and performed a one-way ANOVA for significance, which determined there was no significant difference between the groups (p=0.65, p>0.05). The differences in neurogenesis are shown in Figure 2b.
Figure 2. Doublecortin Stain Data a) Image of a doublecortin stain with the newly formed neuron in bright green and the Hoechst stain in blue b) The results from measuring neurogenesis indicate there is no significant difference between the groups. The error bars represent one SEM.
DISCUSSION.
The data from the Cresyl Violet stain show that there was no significant difference between the hippocampal volume of the three manipulation groups (Figure 1-b, Figure 2-b). Since a high fat diet and the combination of a high fat diet and sleep deprivation have been shown to decrease hippocampal volume in adolescence, our data suggests that there is time to rebound in adulthood [6]. Similarly, the data from the doublecortin stain show that there was no significant difference between the level of neurogenesis between the three manipulation groups (Figure 2b). Since neurogenesis during adolescence has shown to decrease if rats were given a high fat diet or sleep deprivation, our data suggests that the amount of neurogenesis will increase in adulthood.
Neurogenesis and hippocampal volume are both indicators of depression and memory loss [5]. Past studies have shown that rats given a high fat diet have shown depressive behavior and memory loss, but they both rebound in adulthood [6]. Our research shows that the same effect is true for a combination of a high fat diet and sleep deprivation. The rodent model could apply to human teenagers, since rodents have a similar brain structures to people. Many adolescents undergo sleep deprivation and eat a high fat diet and suffer depression and a worse memory [2,4]. However, there are limitations to the rodent model sine the hippocampus of humans is larger and takes longer to develop. Our data suggest that the hippocampus can rebound in adulthood to reverse these effects.
Future studies would include finding the age at which hippocampal structure begins to rebound. Using rats with a wider range of ages could trace the progression of hippocampal volume and neurogenesis as the rats become older. This study could apply to how long it takes for human brains to develop, but a study on people would be necessary to predict exact times it takes to rebound for humans.
ACKNOWLEDGMENTS.
I would like to thank the Belmont SURFs program, for the research space and support. I would also like to thank Dr. Jonathan Creamer and Dr. Jim Dickens from Montgomery Bell Academy for their help through the research process.
REFERENCES.
- Anderson, S. E.,The school district role in educational change: A review of the literature. (International Centre for Educational Change, 2003).
- National Sleep Foundation, 2003 Sleep in America Poll. (2003).
- Knutson, K. L., Spiegel, K., Penev, P., & Van Cauter, E., The metabolic consequences of sleep deprivation. Sleep medicine reviews, 11(3), 163-178. (2007).
- Jacka, F. N., Kremer, P. J., Berk, M., de Silva-Sanigorski, A. M., Moodie, M., Leslie, E. R., … & Swinburn, B. A., A prospective study of diet quality and mental health in adolescents. PloS one, 6(9), e24805. (2007).
- Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V., & Cameron, H. A., Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biological psychiatry, 82(12), 914-923 (2007).
- Boitard, C., Parkes, S. L., Cavaroc, A., Tantot, F., Castanon, N., Layé, S., Tronel, E., Pacheco-Lopez, G., Coutureau, E., & Ferreira, G. adolescent high-fat diet to adult control diet restores neurocognitive alterations. Frontiers in Behavioral Neuroscience, 10,(225). (2016).
- Hulshof, H. J., Novati, A., Sgoifo, A., Luiten, P. G., den Boer, J. A., & Meerlo, P., Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behavioural Brain Research, 216(2), 552-560. (2011).
Posted by John Lee on Wednesday, December 23, 2020 in May 2020.
Tags: Adolescence, High Fat Diet, Hippocampal Volume, Neurogenesis, Sleep Deprivation