Analyzing the Effects of IFN-γ-Induced Repolarization on Bone Marrow Derived Macrophage Viability
ABSTRACT
Macrophages, a key cell in the innate immune system, exist across a spectrum of phenotypes with varying functions. The ends of this spectrum coincide with pro-inflammatory, anti-tumor functions (M1) and anti-inflammatory, pro-tumorigenic functions (M2). Macrophage phenotype can be determined based on changes in cytokine production and surface receptor expression. An inflammatory cytokine, interferon- (IFN-
), has been previously determined to repolarize macrophages from the M2 phenotype to the M1 phenotype. This IFN-
induced phenotypic shift from M2 to M1 is a promising potential immunotherapy for cancer treatment. In this study, we aimed to determine if IFN-
compromised the viability of M2 and M1 macrophages. Viability assays were conducted on M2 and M1 macrophages to quantify the effects of IFN-
at varying concentrations. Murine bone marrow-derived macrophages (BMDMs) were differentiated into M2 and M1 macrophages before being incubated with 0 ng/mL (control), 100 ng/mL, or 500 ng/mL of IFN-
. Additionally, E0771 breast cancer cells were treated with IFN-
at the same concentrations to determine any viability loss in non-immune cells. Results suggest that IFN-
does not significantly compromise the viability of M1 macrophages or E0771 cancer cells. However, for M2 macrophages, at 500 ng/mL there was a significant decrease in viability compared to control, suggesting that this concentration is detrimental. These results provide preliminary data for future experiments involving injectable IFN-
integrated alginate cryogels. Cryogels are promising constructs for the repolarization of M2 macrophages to M1 as a cancer immunotherapeutic delivery system.
INTRODUCTION.
The immune system’s role within an organism is to track down and eliminate any infection to ensure the continuity of the organism’s health [1]. One such component, the innate immune system, supplies the organism with the initial protection against infection and other diseases [2]. Macrophages, a group of immune cells, are crucial components of the innate immune system. Some macrophages, specifically bone marrow-derived macrophages (BMDMs), leave the bone marrow as monocytes, circulate to the tissue, and then mature into macrophages. The primary role of macrophages within a living organism is to phagocytize microbial pathogens and cellular debris [3]. Macrophages are also involved in the regeneration and repair of damaged tissue, helping wound healing and rebuilding the immune system’s barriers after injury [4]. Two distinct macrophage phenotypes have been determined to encompass these aspects. These phenotypes, M1 and M2 macrophages, possess vastly differing functionality in relation to the immune system. The M1 phenotype is pro-inflammatory, aggressive, highly phagocytic, and tasked with clearing the organism of any present infection [6]. Comparatively, the M2 phenotype is anti-inflammatory and associated with wound healing (e.g., healing cuts) [3,4].
In addition to macrophages’ role in the immune system, macrophages have been linked to cancer. Macrophages’ role in cancer has been determined to have multiple aspects in both tumor suppression and inhibition [5]. The two distinct macrophage phenotypes encompass these pro and anti-tumor aspects. The M1 and M2 phenotypes possess vastly differing functionality in relation to tumor cells. The M1 phenotype’s pro-inflammatory nature causes it to be anti-tumor and associated with tumor suppression. Comparatively, the M2 phenotype’s anti-inflammatory aspects are associated with promoting tumor cell proliferation and metastasis [3,4]. Through the induction of an inflammatory response, M1 macrophages are able to decrease tumor growth and metastasis by directly endocytosing tumor cells and inducing an anti-tumor immune response [3]. M2 macrophages, however, counteract this by supplying the necessary signals to continue to drive tumor growth. In most cancers, M2 macrophages are present in the tumor microenvironment, indicating a link between the M2 phenotype and cancer [7]. Specifically, in breast cancer, macrophages have been found to promote tumor growth and metastasis [8]. This abundance of M2 macrophages at the tumor site also indicates a poor prognosis for breast cancer patients [9].
Interestingly, macrophages undergo a phenotypic shift in response to specific pro-inflammatory cytokines, causing a change in macrophage properties. Pro-inflammatory cytokines such as interferon-γ (IFN-γ) promote inflammatory properties in macrophages and are involved in the polarization of M2 macrophages. IFN-γ interacts with receptors on the surface of macrophages initiating internal signals necessary in the induction of a pro-inflammatory response [3]. Thus, IFN-γ is an integral component of macrophage functionality in the tumor suppressive inflammatory response. IFN-γ has also been identified as a target for the development of novel immunotherapies. The phenotypic change in macrophages due to IFN-γ induction also shifts macrophage roles. The previously pro-tumor, anti-inflammatory, M2 macrophages are re-polarized to the anti-tumor, pro-inflammatory M1 macrophages. The re-polarized macrophages can effectively produce an inflammatory response and attack the tumor cells [3,4,5]. These IFN-γ-induced events in macrophages are proposed to be used as a potential cancer immunotherapy in IFN-γ-integrated tough alginate cryogels. Previous studies have demonstrated the efficacy of these cryogels as cancer vaccines [10]. This cryogel scaffold can recruit M2 macrophages into the scaffold to be exposed to IFN-γ. This exposure can effectively re-polarize the macrophages from M2 to M1, allowing an inflammatory response to be promoted against the developed tumor cells.
In this study, we examined the effects of IFN-γ on the viability of a cultured macrophage population in vitro. We expected to see that IFN-γ does not compromise the viability of M1, M2, or E0771 cells. Cell populations included M1 and M2 murine BMDMs in addition to murine E0771 breast cancer cells. The E0771 cell line was tested to determine any decrease in viability in non-immune cells. The collected data provides a basic understanding for future studies involving the repolarization of M2 macrophages to M1 using an IFN-γ treatment. Ultimately, these results will provide insight on IFN-γ treatment as a potential immunotherapeutic option.
MATERIALS AND METHODS.
BMDM Extraction and Counting.
Murine monocytes were collected from the bone marrow of a sacrificed mouse to be later differentiated into BMDMs used in IFN-γ treatments. Monocyte differentiation into macrophages was previously characterized by measuring the expression of F4/80, a surface receptor only expressed on mature macrophages and not monocytes, using flow cytometry [11]. Briefly, the femur and tibia of the mouse were removed and cleaned of connective tissue. The bones were then washed in 70% ethanol for sterilization. 4 total bones (2 femurs and 2 tibias) were collected. The ends of the bones were then cut to expose bone marrow. A 5 mL syringe and 25G needle were then used to flush 5 mL of Dulbecco’s Modified Eagle Media (DMEM) through each bone. The flushed marrow was collected in a 50 mL conical tube containing 10 mL of fresh DMEM. This process was repeated for all bones. Cells were aspirated to break up any cell aggregates and then centrifuged for 5 minutes at 1000 xg. The supernatant was aspirated from cells. The cells were resuspended in 2 mL ACK Lysis Buffer and incubated for 2 minutes on ice. The ACK Lysis Buffer was diluted with 20 mL warm DMEM and centrifuged for 5 minutes at 1000 xg. The cells were resuspended in 10 mL of fresh L929-conditioned BMDM media. The cells were counted by mixing10 μL of the cell suspension with 10 μL of Trypan blue dye. 10 μL of the mixture was then added to a cell counting slide. An automatic cell counter determined total cell numbers. Bone Marrow Extraction/BMDM Counting.
BMDM Plating and IFN-γ Treatment.
The collected monocytes were plated in a 96-well plate at 100,000 cells per well in 100 μL of L929-conditioned BMDM media. At this stage, the monocytes were differentiated to BMDMs. The BMDMs were then polarized to an M1 or M2 phenotype to establish macrophage plasticity to shift between phenotypes. This was done by combining IL-4 and IL-13 for M2 and IFN-γ and LPS for M1. The M1 and M2 macrophages were then treated with free IFN-γ in media at concentrations of 100 ng/mL or 500 ng/mL for 24 hours. To verify macrophage polarization, BMDMs were treated with the appropriate cytokines and then examined with flow cytometry for expression of CD206 and CD86. CD206 is the macrophage mannose receptor overexpressed on M2 macrophages while CD86 is a receptor overexpressed on M1 macrophages [11]. Viability was then examined using a CellTiter-Glo luminescence assay. Luminescence was measured using In vivo Imaging System (IVIS) microscopy to quantify viability.
E0771 Plating and Counting.
Old DMEM media was aspirated off the E0771 cells to prepare for replating in a 96 well plate. Cells were washed with 2 mL of PBS to remove dead cells. The PBS was aspirated off and 2 mL of 0.25% Trypsin was added. The cells were incubated for 5 minutes. We confirmed that Trypsin had broken cell adherence by viewing under the microscope. Excess media was added to wash cells off for collection in a conical tube. The cells were centrifuged at 1500 rpm for 5 minutes. The old media was aspirated, and fresh media was added. Cell counting was repeated. Cells were then plated in a 96-well plate at 100,000 cells per well in 100 μL of DMEM media.
E0771 IFN-γ Treatment.
After E0771 cells had been plated, IFN-γ was introduced to the culture. IFN-γ was incubated with the cells at 100 ng/mL or 500 ng/mL for 24 hours. After 24 hours, we repeated the CellTiter-Glo assay to quantify viability.
Statistical Analysis.
Data is presented as mean ± standard error of the mean (SEM). A one-way ANOVA with Tukey’s post-hoc test was used to compare all groups to each other. To establish statistical significance, we used *p<0.05.
RESULTS.
M1 BMDM IFN-γ Viability.
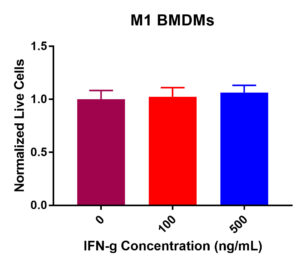
M1 BMDM viability was not drastically decreased post IFN-γ exposure (Figure 1). Some fluctuation occurred across the three experimental values. Results determined that at 0 ng/mL, 100 ng/mL, and 500 ng/mL, viability did not significantly decrease. The normalized live macrophage counts remained constant at around 100K cells per well from 0 ng/mL (control) to 100 ng/mL and 500 ng/mL. Collectively, these data suggest that IFN-γ treatment does not decrease viability as the concentration increased.
Figure 1. M1 macrophage viability in response to IFN-γ incubation at 0, 100, and 500 ng/mL. After 24-hours, no significant change in viability was present. Viability remained largely constant across the experimental values.
M2 BMDM IFN-γ Viability.
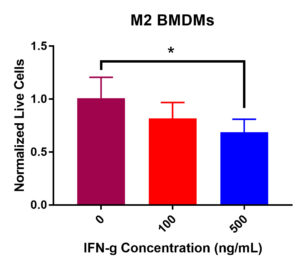
M2 BMDM viability data did display a significant decrease in viability after IFN-γ treatment (Figure 2). Statistical analysis demonstrated a significant decrease in viability from 0 ng/mL to 500 ng/mL of IFN-γ. Additionally, there was an insignificant decrease in viability from 0 ng/mL to 100 ng/mL. Furthermore, normalized live macrophages decreased from the control value of 100K cells per well as IFN-γ concentration increased. Overall, these data suggest that IFN-γ incubation has detrimental effects on M2 BMDM viability.
Figure 2. M2 macrophage viability post IFN-γ incubation at 0, 100, and 500 ng/mL. After 24-hours, a significant decrease in viability was recorded from 0 to 500 ng/mL (*p < 0.05). An insignificant decrease in viability was present from 0 to 100 ng/mL and 100 to 500 ng/mL.
E0771 IFN–γ Viability.
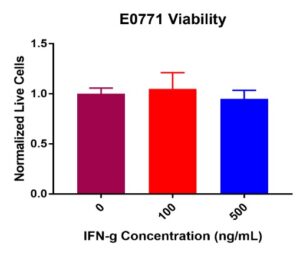
E0771 breast cancer cell viability did not decrease post IFN-γ treatment (Figure 3). No significant decreases in viability were present from 0 ng/mL (control) to 100 ng/mL or 500 ng/mL. Like M1 BMDMs, some fluctuation occurred across the treatment concentrations. Normalized live E0771 values remained constant from the control value of 100K cells per well at 100 ng/mL and 500 ng/mL of IFN-γ. Combined results suggest that IFN-γ does not compromise E0771 viability.
Figure 3. E0771 breast cancer cell viability post IFN-γ incubation at 0, 100, and 500 ng/mL. Results after 24-hours displayed showed no significant decrease in viability across all treatment concentrations. Some insignificant variability was present across treatment concentrations.
DISCUSSION.
The evaluation of M1 BMDMs determined that no significant decrease in viability was present. As displayed in Figure 2, viability remained largely constant across the IFN-γ treatment concentrations (100 and 500 ng/mL). This is to be expected as M1 BMDMs are regularly exposed to IFN-γ as it is essential in the induction of the macrophage inflammatory response [3]. The normalized macrophage cell population also remained largely constant across the IFN-γ incubation values (remained at ~100K cells per well). These data are encouraging for IFN-γ re-polarization in future experiments since M1 viability did not decrease. Comparatively, the assessment of IFN-γ viability on M2 BMDMs demonstrated a significant decrease in viability levels at 500 ng/mL compared to the control. As shown in Figure 3, the results revealed that a statistically significant decrease in viability was present from 0 ng/mL (control) to 500 ng/mL (*p>0.05). Another viability decline, although insignificant, was observed from 0 ng/mL (control) to 100 ng/mL. Additionally, the initial normalized live macrophage population of 100K cells per well (control) decreased to ~80K cells per well at 100 ng/mL and decreased to ~70K cells per well at 500 ng/mL. Combined, these data suggest that IFN-γ significantly compromises M2 phenotype macrophages’ viability at the higher concentration. Due to this significant decrease in viability, the efficacy of IFN-γ as a tool for M2 BMDM re-polarization is questionable at the highest concentration. Further experimentation is necessary to clarify this point, but initial results indicate concentrations below 500 ng/mL should be used moving forward. E0771 viability data also indicated that no statistically significant decrease was present at any of the IFN-γ treatment concentrations as shown in Figure 4. Furthermore, the normalized cell population remained largely constant at 100K cells per well across the treatment values. Together, these data suggest that IFN-γ does not compromise E0771 viability. This information is encouraging as we would not want to see IFN-γ decreasing viability in non-immune cells after treatment.
Due to the viability decrease observed in M2 BMDMs, further experimentation is necessary to determine why this decrease is present. It is suspected that the current IFN-γ concentration of 500 ng/mL is too high to effectively treat M2 BMDMs. Thus, experimentation to determine if lower concentrations of IFN-γ also decrease viability is necessary. Ideally, these lower concentrations would allow viability to remain stable and not decrease. If the lower concentrations also decrease M2 BMDM viability, usage of a different inflammatory cytokine may be necessary. Other potential inflammatory cytokines include: IL-12, IL-17, and IL-23 [3]. Due to the relative constancy of M1 and E0771 viability, it can be determined that these cells can be exposed to IFN-γ without any adverse effects on viability. These findings are encouraging for future experiments involving IFN-γ]. For future studies involving tough alginate cryogels, the data collected in this study provides a basic understanding of how IFN-γ will interact to the cells exposed to the cryogel scaffold. This understanding of how IFN-γ will affect M1 BMDMs, M2 BMDMs, and E0771 breast cancer cells drives the determination of the IFN-γ concentration that will be integrated into the cryogel to successfully re-polarize M2 BMDMs without compromising their viability.
ACKNOWLEDGMENTS.
The author thanks the Giorgio Lab and Dr. Gennifer Goode for their guidance and support. The author acknowledges the Interdisciplinary Science and Research Program at Hillsboro, the Research Experience for High School Students, and Dr. Todd Giorgio for the research opportunity. Additionally, the author thanks Evan Glass and Shirin Masjedi for their continued support.
REFERENCES.
- B. Nicholson, The immune system. Essays in Biochemistry. 60, 275-301 (2016).
- Zhang, J. Chen, Current status and future directions of cancer immunotherapy. Journal of Cancer. 10, 1773-1781 (2018).
- Weagel, C. Smith, P. G. Liu, R. Robison, K. O’Neill, Macrophage polarization and its role in cancer. Journal of Clinical & Cellular Immunology. 6, 1-8 (2015).
- A. Wynn, K. M. Vannella, Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44, 450-462 (2016).
- Keener, Macrophages play a double role in cancer. The Scientist. 2018.
- Ley, M1 means kill; M2 means heal. Journal of Immunology. 199, 2191-2193 (2017).
- B. Hao, M. H. Lü, Y. H. Fan, Y. L. Cao, Z. R. Zhang, S. M. Yang, Macrophages in tumor microenvironments and the progression of tumors. Journal of Immunology Research. 2012, 1-11 (2012).
- Q. Siu, S. J. H. Waaijer, M. C. Zwager, E. G. E. de Vries, B. van der Vegt, C. P. Schröder, Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treatment Review. 70, 178-189 (2018).
- A. Ortega, W. J. Barham, B. Khumar, O. Tikhomirov, I. D. McFadden, F. E. Yull, T. D. Giorgio, Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages. Nanoscale. 7, 500-510 (2014).
- Shih, S. O. Blacklow, A. W. Li, B. R. Freedman, S. Bencherif, S. T. Koshy, M. C. Darnell, and D. J. Mooney, Injectable, tough alginatecryogels as cancer vaccines. Advanced Healthcare Materials. 7, 1-13 (2018).
- B. Glass, S. Masjedi, S.O. Dudzinski, A.J. Wilson, C.L. Duvall, F.E. Yull, and T.D. Giorgio, Optimizing Mannose “Click” Conjugation to Polymeric Nanoparticles for Targeted siRNA Delivery to Human and Murine Macrophages. ACS Omega. 4, 16756-16767 (2019).
Posted by John Lee on Wednesday, December 23, 2020 in May 2020.
Tags: IFN-γ, Immunology, M1 macrophages, M2 macrophages, Macrophages, Repolarization, Viability