Research
Chemical Biology of DNA Damage:
Synthesis, Replication, Repair, and Structure of Carcinogen-Modified DNA
The Rizzo laboratory studies the chemical mechanism by which electrophiles react with DNA to form DNA adducts and their subsequent enzymatic processing (replication and repair). We utilize the tools to synthetic chemistry to incorporate the lesions of interest into oligonucleotides in a sequence-specific manner. We then utilize the tools of biochemistry to examine how a particular lesion affects replication fidelity by DNA polymerases and the recognition of the lesion by DNA repair proteins. NMR and crystallographic methods are used in collaboration with other laboratories to determine the structural perturbations caused by the lesions. The relationship between structure and DNA processing is a central theme of the research program. Current projects involve DNA lesions derived from heterocyclic aromatic amines found in cooked meats, endogenous bis-electrophiles from lipid peroxidations (acrolein and 4-hydroxynonenal) and the degradation of carbohydrates (methylglyoxal), and DNA damaging agents used in chemotherapy (methylating agents and nitrogen mustards). We have a long-standing interest in bis-electrophiles that can form interstrand DNA cross-links and DNA-protein cross-links. The Rizzo laboratory is associated with the NIEHS supported Center in Molecular Toxicology (of which Prof. Rizzo serves as Co-Deputy Director and Leader of the DNA Damage and Genetic Instability Research Core), the NCI supported Vanderbilt-Ingram Cancer Center, and the Vanderbilt Institute for Chemical Biology.
Heteroaromatic Amines. 2-Amino-3-methylimidazo[4,5-f]quinolone (IQ) is a heterocyclic aromatic amine formed in cooked meats as a result of Maillard chemistry. After bioactivation, IQ modifies DNA to give a major C8-IQ-dG and minor N2-IQ-dG adducts. IQ is a potent inducer of two-base frameshift deletions in CG dinucleotide repeat sequences in bacterial mutagenesis assays. Our lab was the first to synthesize oligonucleotides containing the C8-IQ-dG and N2-IQ-dG adducts, which allowed us to examine the conformation and replication of the adducts.
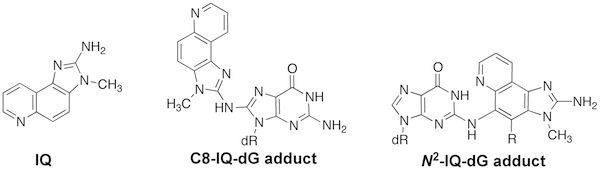
We initially characterized the C8-IQ-dG adduct using UV and CD spectroscopy and thermal melting analysis (Tm). We concluded that the C8-IQ-dG adduct adopted a base displaced intercalated conformation when situated in a frameshift-prone CG repeat sequence and was groove-bound in a non-repeat sequence. These conclusions were subsequently confirmed by full NMR structural analyses by the Stone Lab.
The replication of the C8-IQ-dG and N2-IQ-dG adducts was examined in vitro with a panel of prokaryotic and eukaryotic DNA polymerases in collaboration with the Guengerich lab. We observed that replication of the C8-IQ-dG adduct in the CG-repeat sequence resulted in frameshift deletions for the prokaryotic polymerases, whereas replication in the non-repeat sequence resulted in error-free bypass. The N2-IQ-dG adduct was a strong block to replication. Of interest, bypass of the C8-IQ-dG adduct with human polymerases did not give deletions; however, replication of minor N2-IQ-dG adduct by human pol η resulted in -2 deletions in the CG-repeat sequence. These observations help explain mammalian mutagenesis data of arylamines (including IQ), which reported that frameshifts were the minor mutagenic event. This is because the N2-IQ-dG adduct is the minor DNA adduct and will only give deletions in CG repeat sequences.
Formamidopyrimidine Lesions. Formamidopyrimidine (Fapy) lesions result from fragmentation of the imidazole portion of purine bases. These understudied lesions can arise from either oxidative damage to dG or dA or initial N7-alkylation of dG. An interesting and unusual feature of Fapy lesions is that can exist as either the natural β- or unnatural α-anomers. Previous reports suggest that the methyl-Fapy-dG lesion is repaired less efficiently than other methylated DNA adducts including O6-methyl-dG. We developed a general, solid-phase approach for the site-specific synthesis of oligonucleotides containing N5-substituted Fapy-dG lesions derived from methylating agents, chlorooxirane (the metabolically activated form of the occupational toxican vinyl chloride), and nitrogen mustards which allows us us to examine their replication and repair.
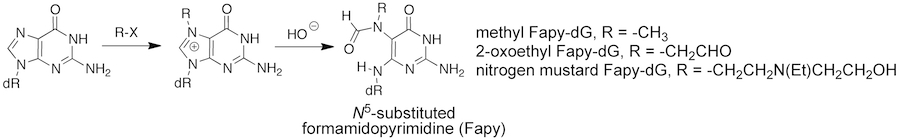
We are particularly interested in the biological processing of the methyl- and nitrogen mustard Fapy-dG lesions since these are possible cytotoxic lesion cause by chemotherapuetic agents (i.e. temozolomide and mechlorethamine). Repair and replication of these lesion represents mechanisms of drug resistance. Further, error prone replication of these lesions is likely to be an inititating event for the formation of secondary cancer, which often results from chemotherapeutic alkylating agents. Understanding the replication and repair of these lesion may provide new targets for combination therapies to improve the clinical efficiancy of these agents.
DNA Adducts Derived from Endogenous bis-Electrophiles. Oxidative damage to lipids and carbohydrates produces a wide array of electrophilic species that react with proteins and nucleic acids. We developed chemistry to incorporate these adducts into oligonucleotide and discovered that form DNA interstrand cross-links (ICLs) and DNA–peptide cross-links (DPCs). The cross-linking was highly dependent on stereochemistry in the case of the crotonaldehyde and 4-HNE adducts. Our laboratory conceived of NMR methods to follow the cross-linking chemistry by NMR, which invovled incorporating specific 15N and 13C labels into the adduct and complementary oligonucleotide. These studies are of particular interest since ICLs are regarded as a very severe forms of DNA damage. Individuals with the rare genetic disorder Fanconi anemia show a significant predisposition to cancer. Fanconi anemia cells are hypersensitive to DNA cross-linking agents, suggesting cells have evolved critical ICL repair pathways as protection against bifunctional electrophiles. The significance of our observations is underscored by recent studies demonstrating the repair of DNA damage derived from endogenous aldehydes is dependent on the Fanconi anemia pathway.
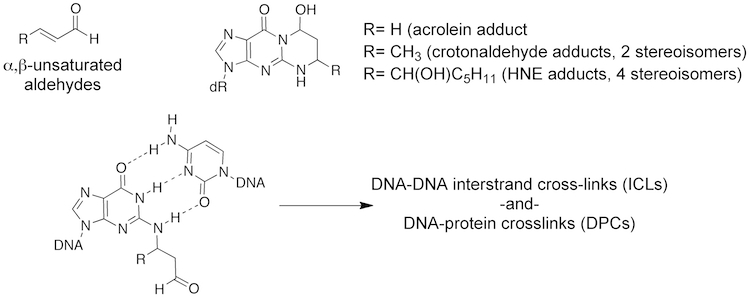
In collaboration with the Lloyd laboratory, we discovered a potential damage tolerance pathway in which human pol κ can replicate past an ICL in an error-free manner. Nitrogen mustards were the first clinically used cancer chemotherapeutic agents and their mechanism of action involves the formation of ICLs. This observations suggests that pol κ may be part of a resistance mechanism to nitrogren mustards and inhibitors of pol κ in combination with nitrogen mustards may be a more effective chemotherapeutic regimen.