Q_A_2012
Thank you for your thoughtful questions
Chapter 13: Spectroscopy
Q: When do complex splitting (i.e., triplet of doublets) simplifies. When are the coupling constants similar enough to simplify? For example, my TA in recitation said that protons in alkanes generally have close enough coupling constants so we can simplify them. What about phenyl protons?
A: The multiplicity simplifies when the coupling constants are the same. Consider the case when two sets of non-equvalent protons (A and C) and coupled to a common set of protons (B). Protons A and C will couple to B independently and a complex splitting is observed when J(AB) is not equal to J(CB). However, if J(AB) = J(CB), the observed multiplicity is as though protons A and C are equivalent. It is difficult to predict coupling constants precisely enough to know when they will be equal. You need to aware of the possibility in order to interpret a spectrum properly. The coupling constant is related to the dihedral angle between the H’s. Because there is free rotation about aliphatic chains, the dihedral angle between the H’s tend t average out and hence the coupling constants are the same.
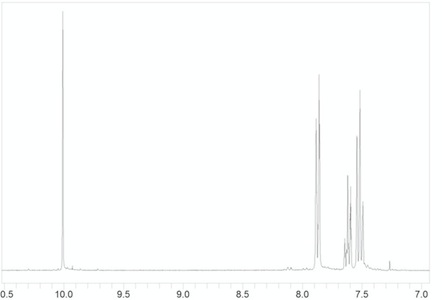
Yes, it can happen with aromatic rings. Attached is the NMR spectrum of benzaldehyde. As a mono substituted aromatic we expect three aromatic resonances that will have a 1:2:2 ratio. The para proton has two equivalent protons adjacent and should appear as a triplex (d=7.6 ppm). The ortho protons have one proton on the adjacent carbon, so it is a doublet (d=7.85). The meta protons have two non-equivalent protons on the adjacent carbons, so it should appear as a doublet of doublets – however, in the actual spectrum it looks like a triplet (d=7.5).This is because the two coupling constants are the same.
The multiplicity of aromatic protons is generally more difficult to interpret. Benzaldehyde is a useful example because the three resonances are sufficiently spread out. Many time the aromatic protons fall on top on one another. The multiplicity of aromatic protons is also complicated by 4 bond coupling, which is more common in aromatic ring, and by magnetic non-equivalence, which we do not discuss.
Q: I am slightly confused by the section on the M/M+1 differentiation. Am I correct in thinking that the M + 1 is the isotope that is just one proton “heavier(?)” than the most abundant isotope? I think that I understand the general idea, that if Cl 35 was M then Cl 37 would be M + 2, I just don’t particularly understand the reasoning behind why that is.
A: An isotope of an element has extra neutrons – protons and neutrons are both ~ 1 atomic mass unit. Yes, an MS spectrum of an organic compound will show an M+1 peak because some of the molecules wll contain 13C. In the case of Cl, the most abundant isotopes have atomic masses of 35 and 37, so a chlorine containing molecule with have an M+2 peak.
Q: I am looking at the old exam (Question 8), and am confused as to why all three of the methods for preparing t-buytl ethyl ether are valid because I have in my notes that tertiary alkyl halides DO not undego SN2 reaction. So I was just wondering how this could be a feasible method for preparing an ether even though there are few limitations I specifically have that written down.
A: It is a mistake – the answer should be C
Q: I am confused about the mechanism for the reduction of a ketone or aldehyde. In my notes, I treated NaBH4 as H- when doing my mechanisms, having the H- attack the carbon of the ketone/ aldehyde. However, the mechanism presented in the book is slightly different with the bond between B-H attacking the carbon and then the B forming a complex with 3 of the ketones. Which one is correct?
A: Mechanism 15.1 (page 653) in the book shows the hydride being delivered to the carbonyl carbon by BH4-; I said the BH4- (asm well as LiAlH4) can be thought of as H-, and H- adds to the carbonyl carbon. So this part is equivalent. The book shows BH3 acting as the counter ion to the O- of the reduced carbonyl. I neglected the counterion and simply represented this as O- this as O-. Please keep in mind that the counter ion is important, abd thinking of NaBH4 and LiAlH4 as H- is a simplification. As I noted in class, NaBH4 and LiAlH4 are nucleophilic hydride sources (H-) for the reduction of carbonyls. However, NaH and KH, which are also hydrides, do not reduce carbonyls.
Q: In class we talked about the mechanism of the acid-catalyzed condensation of an alcohol to ethers. In my notes I have that the acid (H-A) is regenerated after A- abstracts a proton. In the book (mechanism 15.2), I’m confused as to why sulfuric acid is not regenerated. The mechanism shows that ethanol (and not the hydrogen sulfate ion) abstracts a proton from the diethyloxonium ion in step 3. Why is this?
A: This is equivalent. I like to show A- abstracting the proton from the protonated ether (diethyloxonium ion) and discretely show that the acid catalyst is regenerated.In this case, the acid H-A re-enters the catalytic cycle at step 1. However, as the book shows, a third molecule of ethanol to pick up the proton to give protonated ethanol, which re-enters the mechanism in step 2. So the catalytic cycle is maitained.