Q_A_2006
Thank you for your thoughtful questions
Q: Will the Final exam emphasize the chapters 26-28 or will it also be more of a comprehensive final?
A: It will be comprehensive. My intent is that all the chapters will be equally represented on the final. There are overlapping themes in many of the chapters, i.e., Chapters 15 – 16 and part of 24 (electrophilic substitution), and chapters 19-23 (carbonyl chemistry). Themes somtimes reappear in subsequent chapters with expanded scope or emphasis (amine synthesis and amino acid synthesis). So the themes may be represented more so than the individual chapters.
Q: Will you be offering the alternate final exam on Wednesday, May, 3rd?
A: As stated on the syllabus, there will be no alternative final exam offered.
Q: Just to clarify, we do not need to know the amino chart in the beginning of chapter 26, right?
A: You do not need to know the structures amino acids per se (by popular demand), but you should be able to identify a hydrophobic, hydrophilic, basic and acidic amino acid when you see one.
Q: I was also unsure as to whether or not specific structure knowledge for portions of chapter 27 (i.e., phospholipids, prostaglandin, etc) would fall under the same category as the amino acid table.
A: As with the amino acids, you do not need to know specific structures, but you should be able to identify specific classes of compounds.
Q: On slide 369 we have a heading for section 26.9 with the (please read) caption, which we really know is secret code for “you will not be tested on this.” Slide 370-377 then seem to fit under this heading. Are we to assume the material from slide 370-377 really do fall under the same caption as 26.9?
A: la·gniappe, [lan’ yap] n, something given or obtained gratuitously or by way of bonus or good measure; a small gift given to a customer.
Chapter 28
04-27-2006
Q: I believe that the solutions manual made a mistake in synthesizing 5′-CTAG- 3′ (Problem 28.49). I believe they actually made 3′-CTAG-5′.
A: You are correct. They synthesized the the DNA backwards. Be sure to remember that the chemical synthesis of DNA and peptides is done in the opposite deirection as the convention for writing a sequence.
Q: Why is chemical DNA synthesis done 3′ to 5′?
A: The primary 5′-hydroxy group is less hindered and therefore more nucleophilic than the secondary 3′-hydroxy group.
Q: On slide 443: why does DMT-Cl react w/ the 5′-OH and not the 3′-OH?
A: For the same reasons as above. The DMT group is pretty big and reacts much faster with less hindered primary alcohols (5′-OH) than more hindered secondary alcohols (and not at all with tertiary alcohols)
Q: How can diisopropylamide act as a leaving group when it is the conjugate base of an extraordinarily weak acid? (p. 1084, step 3 of DNA synthesis)
A: The pKa of tetrazole is about 4.8. Tetrazole is an activator in this reaction and protonates the nitrogen of the phosphoramidite. This makes the diisopropyl ammonium a much better leaving group.
Chapter 26
04-24-2006
Q: Why do we use acetamidomalonate (with the amide group) rather than aminomalonate (with an amine)? The amide is ultimately hydrolyzed to the amine, but why begin with the amide?
A: A free amino group is nucleophilic and would compete in the SN2 reaction with alkyl halides.
Q: Why use the cyclic acetal protecting group during enantioselective catalytic hydrogenation (slide 359)?
A: It simultaneously protects both hydroxyl groups as acetal of formaldehyde. Protecting 1,2-diols as acetals or ketals is standard.
Q: Could you provide the mechanisms for the penultimate and ultimate steps of the derivatization with ninhydrin (slide 364)?
A: The penultimate step is the hydrolysis of an imine. So it would be the reverse mechanism as the formation of an imine (Chapter 19.9). The ultimate step is the formation of an imine with a second equivalent of nunhydrin.
Q: What is the purpose of amino acid analysis (determining the percent composition of each amino acid) if peptide sequencing (via Edman method) provides this information in addition to the linear amino acid sequence?
A: Sequencing is done on small fragments. The amino acid analysis is can be done on the entire protein. Since these are large macromolecules, determining structure is much more challenging and the error associated with each method is larger. Amino acid analysis is another piece of data that serves as a check for other methods. In the end, all the data should be reasonably consistent.
A: Could you provide the mechanism for the acid-catalyzed rearrangement of the anilinothiazolinone (ATZ) derivative to an N-phenylthiohydantoin (PTH)?
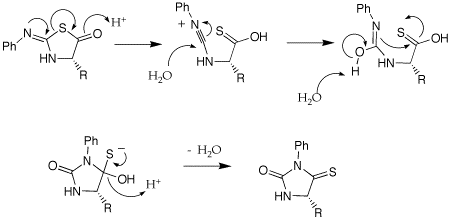
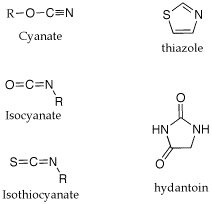
Q: Could you please explain some of the nomenclature used in the Edman degradation? (isothiocyanate, anilinothiazolinone, thiohydantoin).
A: I am not sure where the names come from. The attached gif shouws the structures of the parent ring structures. A cyanate is a R-O-CN; a isocyanate is isomeric in the placement of the R group, O=C=NR. A isothiocyanate substitutes sulfur for O, S=C=NR. The thiazolinone is based on a thioazole ring structure, the -one implies a ketone carbonyl within the ring and the anilino implies a aniline imine as part of the ring structure
Q: I do not understand why the Edman degradation has a length limitation. The book indicates that it is due to the build-up of unwanted byproducts, which I assume refers to the PTH-derivatives (?). If these are eluted from a column, however, does this not remove them from the system, allowing one to continue? What exactly limits this process?
A: The use of the an LC column is for analysis of the reaction not for purification of the product. The peptide and reagents are in a reaction vessel and the PITC is added and the pH adjusted to achieve the N-terminal amino acid cleavage and conversion to the thiohydantoin derivative. An aliquot is removed for the LC analysis and identification of the amino acid derived thiohydantoin. More PITC is then added to the reaction and the pH is cycled to get cleavage of the next amino acid (from the N-terminal end); however, the thiohydantoin from the previous cycle(s) is still part of the reaction mixture. After 20 cycles or so, the analysis become complex.
Q: What is meant by the “orthogonal protecting group strategy” (slide 378).
A: When there is more than one protecting group in a substrate, each protecting can be removed selectively without removing the others. In the case of peptide synthesis it is critical to be able to deprotect the alpha-amino group, without removing the protecting group on the carboxylate.
Q: Why is trifluoroacetic acid used for removal of the BOC protecting group? Could one instead use a mineral acid?
A: Trifluoroacetic acid (TFA) is used neat. Since trace water interfers with the peptide coupling step, it is desirable to avoid water during the peptide synthesis. TFA is actually low boiling and it is easy to get rid of.
Q: On page 1007 (peptide synthesis), why are steps 4 and 5 not combined into one? Both groups could be removed with acid (we learned in ch. 21 that ester groups are hydrolyzed by acid)
A: In principle it can be done in one step; however, aqueous acid is not as effective for removing a BOC group as neat TFA. The removal of the BOC group is actually not a hydrolysis. Q:
Q: Why are fibrous proteins insoluble in water? Do they not have hydrophilic amino acids distributed on their exterior?
A: The structure of fibrous proteins does not optimize the packing of the hydrophobic amino acids into the middle of the structure. I think it has more to do with the hydrophobic amino acids being exposed to the solvent. They also tend to be high molecular weight oligomers.
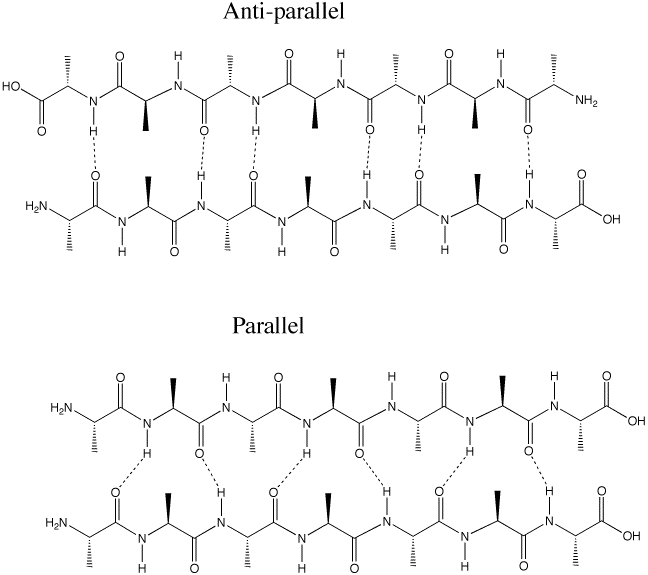
Q :Why is the antiparallel arrangement of the beta-sheet more energetically favorable than the parallel arrangement (p. 1011)
A: The hydrogen bonding between in the anti-parallel beta-sheet is better aligned than in the parallel. Hydrogen bonds are most stable with the O-H-N angle is 180 degrees. This can be achieved in the anti-parallel, but not in the parallel arrangement
04-10-2006
Q: On slide 352 where the basic and acidic amino acids are shown, why would acidic amino acids have more COO- than NH3+ and vice-versa for basic when the COO- is supposed to be more basic and the NH3 more acidic?
A: Both CO2H’s of Asp and Glu are more acidic that the NH3+; so the CO2-‘s would be weaker bases that -NH2. However, the overall charge state will depend on the pH of the solutions (see slide 354 on pI’s; I should have drawn a arrow from right to left for increasing pH). If you are asking why I drew them in the completely ionized forms, it is probably because in peptides and proteins (which is really the more interesting situation), the a-amino carboxylate portions would be involved in amide linkages and are not in the zwitterionic forms. The side chains are largely in the ionic forms at neutral pH.
Exam 3 (04-07-2006)
Q: Will you be providing a “reagents bank” like you did last time? I’m finding that I can easily recognize what each reagent does when I see it, but being able to call up all of the reagents is a bit more challenging.
A: Haven’t decided yet, but it would be safer if you are prepared for a challenge.
Chapter 25
04-28-2006
Q: On p. 948, it appears that the text relates R to D and S to L. Is this correct? I thought there was no relationship between R/S and D/L.
A: It is probably generally true for carbohydrates by virtue of the structural similarities between carbohydrates. However, R/S is a nomenclature system with rules. D/L has a physical meaning in the Fischer projection context. Cysteine is a good example where this breaks down. All of the amino acids are of the L-configuration which pertains to the position the alpha-amino group on the Fischer projection. Cysteine is R, while all the other amino acids are S.
04-06-2006
Q: for Chapter 25, do you want us to memorize the names of all the pentoses and hexoses from section 25.4?
A: I think you should be familiar with glyceraldehyde because all stereochemistry is related to it and it is of historical significance. You do not need to know the names or structures of any other carbohydrates, but you should be able to idenified their classification, i.e., a ketose versus aldose, furnaose versus pyranose, monosaccharide versus disaccharide, reducing versus non-resucing, etc). It would be helpful if you know that -ose means a carbonhydrate.
Q: I’m at the Fischer Proof section now and was wondering what you want us to note from this? I understand how he was able to go through deductive reasoning to figure out structures based off of optical activity, so was this section pretty much just to illustrate how incredibly much reasoning power went into early/current organic chemistry?
A: It was an illustartion of the use of the chain-lengthing reaction (the chain shortening could also have been used) in combination with the use of stereochemistry and stereochemical relationships and optical activity for structure proof.
Q: Why aren’t polysaccharides considered reducing sugars if they have one free anomeric carbon at the end?
A: In principle, a polysaccharide should be classified as a reducing sugar if it is derived from an aldose and has a free anomeric end. Polysaccharides are very large polymers having MW on the order of 500,000 and 3,000 carbohydrate units per molecule of polymer. The reducing end of the polysaccharide is a small fraction of the carbohydrate present. So a 3000 carbohydrate polymer has a single carbohydrate unit which is a reducing end. It is likely that one can’t get enough polysaccharide in solution on a molar basis to get an appreciable Tollins test that is visible.
Q: What is the use of dtermining whether a carbohydrate is a reducing sugar?
A: A reducing sugar gives a positive Tollins test. A carbohydrate gives a positive Tollins test if it can have an ring open form that is an aldehyde.
Q: Can you control whether you get alpha or beta product in disaccharide formation?
A: Only through the Knonigs-Knorr reaction (as well as variants to this reactions) can they be synthesized and the stereochemistry controlled for disacchardides. Nature synthesizes disaccharides and larger carbohydrates and controlls the stereochemistry enzymatically.
For monosaccharides the alpha and beta hydroxyl groups are in equilibrium, so there is no way to control the stereochemistry there.
Q: Is the Koenigs-Knorr reaction different from glycoside formation in any other way besides that it gives only beta product? Also, are there any limitations on the alcohols used in these reactions?
A: What is presented in Chapter 25 on the Koenigs-Knorr reaction is very fairly minimal. On can not make couple two carbohydrates together with acid to get a disaccharide and has to use the Koenigs-Knorr approach. There are restrictions to this reactions but most are specific to the particular reaction that is being done and the specific carbohydrates involved.
Q: Can the Koenigs-Knorr reaction be used for any carbohydrate?
A: In principle it can be used with any carbohydrate. If a 2-deoxysugar is used, the stereochemical preference for the glycoside formation is often lost.
Q: Slide 343: Is the reason that there is no open form of sucrose that both linked carbohydrates are glycosides? If so, how do they link so that the two anomeric carbons are connected? Also, why is the anomeric carbon an fructose number two instead of number one?
A: Yes, when the anomeric position is involved in a glycoside linkage, then it will not have an ring open form.
We really didn’t talk about disaccharide synthesis much. While the anomeric hydroxyl group of the cyclic form has special reactivity, any hydroxyl group of a carbohydrate can be involved in a glycoside linkage.
The anomeric carbon is the one that is a carbonyl in the ring open form. For fructose, which is a ketose, that is carbon 2.
Q: Slide 331: You said in lecture that acetyl chloride could also be use in ester formation, would the product be the same but with chloride in place of the methyl group? When would you want to use one over the other?
A: No, you use acetyl chloride in place of acetic anhydride. They have equivalent reactivity and give the same product.
Chapter 24
04-28-2006
Q: For the Hofmann elimination, is Ag2O/heat OR a strong base like (CH3)3CO-K+ enough to eliminate the amine group?
A: I skipped the stuff about exchanging the counter ion with Ag2O. Treating the trimethylammonium salt with a strong base, such a t-butoxide, will give the Hofmann elimination
04-06-2006
Q: I was wondering if sections of Chapter 21 that we didn’t get to cover for the last test will be on the upcoming exam (if so, which sections are they again?).
A: The material that we didin’t get to in Chapter 21 was on the Chemistry of Amides. Some of that has resurfaced in Chapter 24
Chapter 23
04-06-2006
Q: Regarding slide 263, is the rxn with (H3C)3CO-K+ just a way to remove HBr to make the double bond?
A: Yes, Potassium tert-butoxide is my official E2 elimination base.
Q: In the reaction which gives alpha-beta unsaturated esters, acids and amines, where does the group Y come from and what is it representing? In the same reaction series, is R any ester or any amide? Also, how is R removed and why add it if only to remove it later? Are R and Y originally in the same compund?
A: The first part of the sequence is the HVZ top initally get the a-bromo acid bromide. We saw in Chapter 22.4 (ssee slide 234) that the a-bromo acid bromide can be converted into the a-bromo carboxylic acid, a-bromo-amide or a-bromor ester depedning on how you quench the HVZ reraction. Y represents these various permutations of the HVZ reaction, so Y is weither an -OH, NR2 (generic amide) or OR (generic ester). For the reaction immediately above shows the bromination of a carbonyl group (either an aldehyde or ketone) folloed by elimination to the a,b-unstaurated carbonyl. We only considered reaction at one side of the carbonyl and the R is just a generic organic group (or an H if it is an aldehyde)
Q: In the Claison Condensation, will you please explain how the deprotenation drives the reaction forward?
A: The Claisen reaction is reversible. If you take the b-keto ester product and add ethoxide to the keto carbonyl, you can break the C-C bond to give the ester and the enolate. When the product is deprotonated, the reverse reaction is prevented because ethoxide can not longer add to the negatively charge product. Deprotonation therefore stabilizes the product. Whenever you stabilize one side of an equilibrium, you drive the equilibrium reaction in that direction
04-05-2006
Q: During the aldol reaction, does water leave spontaneously, or is there a specific catalyst/reactant used to remove water and create a C=C double bond?
A: The dehydration occurs with either catalytic acid or base. The dehydration of of the beta-hydroxy-carbonyl product can occur during the aldol reaction if the reaction is done at room temp or above. At lower temperatures, the beta-hydroxy-carbonyl is stable.
Q: On page 875 of the text, the authors state that the first step in the reaction process is enamine formation from a ketone. Can the reacting enamine not be formed from an aldehyde?
A: They can be used with aldehydes but are more commonly used with ketones.
Q: When aqueous acid is added in the final step of the Stork reaction, will esters and nitriles present in the reacting species be hydrolyzed along with the enamine? If not, why? [this pertains to question 23.19 (a) and (c), page 875- the solutions manual indicates that these functional groups are not hydrolyzed]
A: The hydrolysis of nitriles and esters usually requires more vigorous conditions than for the hydrolysis of an enamine or imine back to the ketone (more conc. acid and usually heat). So these functional groups are safe during that step.
04-01-2006
Q: On slide 269 (23.5 Using Aldol Reactions in Synthesis), why is it that you get the b-hydroxy carbonyl or the alpha/beta unsaturated carbonyl from the aldol reaction. Is this the mixture of products that can result (it appears that the unsat. carbonyl is the dehydrated product of the b-hydroxy carbonyl??)?
A: We are not talking about getting a mixture of products although that is certainly a possibility. The aldol reaction can give two possible products. It can be stop at either the beta-hydroxy carbonyl stage. The dehydration can be catalyzed by either acid or base. The aldol reaction is often run under catalytic base conditions. If the aldol is done at lower temperatures, say 0° C or below, then the b-hydroxy carbonyl is the product. If the aldol reaction is run at room temperature or above, then the dehydration occurs top give the a,b- unsaturated carbonyl
Q: When we talk about enolates, are we only talking about enolate ions?
A: Yes, the word enolate refers to the enolate anion.
Q: LDA in THF at -78 degrees helps to cause E2 elimination as a strong base; but it also is used in the alkylation of ketones/esters/nitriles?? I am trying to understand slide 274 where is shows discrete (in situ) generation of an ester enolate with LDA; what purpose is the enolate serving in this purpose? Is it E2 elimination again or alkylation as well?
A: LDA can be used to affect an E2 elimination reaction. However, the way we have used LDA is to perform a-deprotonation of carbonyl compounds. On slide 274, once the enolate is formed it is undergoing a Claisen condensation with various esters. There is no E2 elimination on this slide.
Q: So, does the aldol reaction require that one of the reactants has alpha hydrogens whereas the Claisen condensation can do a reaction between a ketone enolate with an ester to form a 1,3 diketone?
A: The aldol and Claisen reactions are very closely related. Any of the enolates that we have discussed can be used for both of these reactions. The only difference is that the electrophile. For the aldol reaction, the enolate reacts with an aldehyde or ketone to give a b-hydroxy carbonyl (which can dehydrate to the a,b-unsaturated carbonyl). For the Claisen reaction, the enolate reacts with a ester to give a 1,3-dicarbonyl (rather that a 3-hydroxyl carbonyl which is the same as a b-hydroxy carbonyl). There is a parallel to the reactivity. The electrophile for the aldol is a ketone or aldehyde and the product is a b-hydroxy carbonyl. The electrophile for the Claisen is a ester (which is in the next higher oxidation than a ketone or aldehyde , the electrophile for the aldol) and the product after reaction with the enolate is a b-keto carbonyl, which is in the next higher oxidation state than a b-hydroxy carbonyl from the aldol reaction.
Q: On slide 274, how do we know that the Claisen condensation always breaks the bond between the C and the OEt?
A: The Claisen condensation is a nucleophilic acyl substitution reaction between an enolate and an ester. When the enolate adds to the ester, a tetrahedral intermediate is formed. The carbonyl is the re-formed when the EtO(-) is ejected as a leaving group.
Q: On slide 277, what is the purpose of having EtONa and EtOH in the second step when the bromoalkene is added? (what is that reaction?)
A: This is a a-substitution reaction. The substitute reaction of the hydrogen for an alkyl group must go through the enolate. The EtONa is to generate an enolate.
Q: If decarboxylation is a loss of CO2 why does the whole of the CO2Et get removed in the addition of H30+ and heat in the reaction on slide 277?
A: This is a two stage reaction. The first is the acid catalyzed hydrolysis of the ester to the carboxylic acid. The second is the loss of CO2; overall, the CO2Et is lost as CO2 and EtOH
Q: When you were drawing the reaction for the Robinson Annulation you mentioned that of the four alpha carbons/hydrogens one is far more acidic and it was the one between the two carbonyl groups – why was this not the one extracted by EtO-?
A: You are talking about the intermediate after the Michael addition that undergoes intramolecular aldol to give the cyclohexenone product. There are at least four different enolates that are possible from that intermediate. The major enolate from deprotonation between the ketone and ester can react with the other ketone, but this would give a disfavored four member ring aldol product. Deprotonation at either of the methyl groups next to the ketones, gives an enolate that can react with the other ketone to give a much more favored six-membered ring aldol product (see next question). Even though these enolate are present in much lower concentrations, they react to give more stable products, so the aldol to give six-membered ring is more favored than to give the four-membered ring.
Q: Would two products not have been possible from the Robinson Annulation you showed us (for ex. two products with alkene double bond in two different places?)
A: For the Robinson annulation product on slide 283, it is possible for the double bond to be in conjugation with the ester. However, the base catalyzed dehydration of water that yields this double bond results from a-deprotonation and elimination of hydroxide. One would expect deprotonation alpha to the ketone rather than the ester because it is the more acidic position.
If you look at this intermediate on slide 282, the carbon fragments are color coded. What is shown is the blue methyl ketone undergoing aldol reaction with the green ketone and dehydration to give the product. It is possible that the green methyl ketone can undergo aldol reaction with the blue ketone followed by dehydration to give a cyclohexenone was not shown on the slide in the notees (see below). This is certainly a feasible reaction, which I did not consider. Thamks for pointing this out.
03-31-2006
Q: On page 863 (middle of the page), the reaction is a mixed aldol reaction; why does it produced an unsaturated product using the same reagents (NaOEt and ethanol) while the reaction at the end of the chapter on p. 881 (1c) shows the
product not having been dehydrated? (yet, the reactants are still the same)
A: As I noted above, the conditions of the aldol reaction determine if the product is a b-hydroxy carbonyl or the a,b-unsaturated carbonyl. You need to be able to recognize that two products are possible for the aldol reaction.
Q: Is the effect of acetoacetic ester synthesis the addition of three carbons to the carbon chain?
A: Acetoacetic ester is initally a four carbon unit (not including the OR of the ester). After decarboxylation, a three carbon unit is added.
Q: Can cycloalkanes be made with acetoacetic ester synthesis like that can intramolecularly with malonic ester synthesis?
A: Yes
Q: Is the enol-keto tautomerism essentially equivalent to the relationship between resonance structures?
A: No! Enol-keto tautomers there is a movement of a proton. You can only move electron when draeing resonances structure; atoms can not move
Q: In the carbonyl condensation reaction, why do we show the carbonyl as an enolate ion and not an enol?
A: This depends on the on the reaction conditions. If it acid catalyzed, then the enol reacts, but this is less common and less reactive. For the base catalyzed reaction with ketones or aldehydes, you can draw either the enol or enolate- the enolate is more reactive than the enol, so I usually show the enolate reacting. The pKa pf beta-dicarbonyl compounds is low enough that the enolate is the reactive species when treated with base. If you use a strong base such as LDA, the the carbonyl compound is completely deprotonated and the enolate is generated.
Q: Is it true that the starting carbonyl in a carbonyl condensation reaction is an electrophile that than becomes a nucleophile upon attacking the second carbonyl compound?
A: It becomes a nucleophile (and stops becoming a electrophile) upon enolate or enol formation.
Q: Why is the aldol reaction between two ketones the first reaction listed in Chapter 23 if it is so unfavorable? Is it still a reliable reaction?
A: I don’t thing that the way it was presented specified a ketone per se. It could also be an aldehyde. The self-condensation of either a ketone or aldehyde is the basic reaction
Q: In the acid catalyzed dehydration of aldol, it is possible to go directly from the first resonance structure enol to the final product with E2 elimination and bypass two or three steps, right?
A: If you mean to bypass the resonance structure with the the negative charge on the alpha-carbon, yes. Remember, that neither resoancne structure is truly correct- the the actual structure is a hybrid of the resonance structures.
Q: On the last reaction of slide 261, should there be two equivalents of the reactant to do a carbonyl condensation reaction followed by an aldol reaction?
A: The slides numbering has gotten messed up so I am not exactly sure what reaction you are talking about. If it is the aldol reaction of cyclohexanone, then yes, there should be a two in front of the reactant.
Q: Is the carbonyl condensation reaction/mechanism discussed first in Chapter 23 an overall reaction/mechanism for carbonyl compounds or is it its own separate reaction? It was not listed in the summary of reactions at the end of Chapter 23, so I was just wondering what its status was.
A: The reaction shown on page 855, Fig 23.1 is the base-catalyzed aldol reaction. It is the general reaction that is listed at the aldol reaction in the Summary on page 881. It happens to be listed as three specific types.
Q: What were the conditions for that you gave in slides 262 and 263 (for ex. if one partner has no alpha H or if one of the carbonyl species is way more acid than the other)? Were they conditions for producing one product in the mixed aldol reactions as opposed to 4?
A: The mixed aldol reaction (slide 264 in the latest version) gives four possible products. This uses the base-catalyzed conditions shown. The way to get just one is to discretely generate the enolate from one of the aldehydes (lets say pentanal) with LDA in THF at -78° C; this completely convert pentanal into the enolate. After the enolate is formed, the other aldehyde is added (in this case propanal). This will give just one of the aldol products: 2-(1-hydroxyproyl)penanal (top right) because the pentanl was convereted to the enolate which is going to act as the nucleophile and propanal is the electrophile in the reaction.
Chapter 22
04-06-2006
Q: When, if ever, is the haloform reaction useful?
A: There are a few examples in the book when a methyl ketone is converted to a carboxylic acid (by loss of the methyl group through the iodoform reaction). Overall, this is not a useful reaction
Q: In the malonic ester and acetoacetic ester syntheses, the products are an alkylated carboxylic acid and an alkylated methyl ketone, repectively.
a. Is it correct that you first make an enolate ion with LDA (or another base) and then react it with an alkyl halide and then use acid to get the final product?
A: The text did not emphasis the formation of the enolate with LDA, although this is the most reliable way to form enolates. The alkylation step can be done with catalytic base. The decarboxylation of the alkylated product is done with acid treatment.
Q: b. Could you, and if so would you, ever stop after the reaction with the alkyl halide?
A: It depends on the goals of the synthesis. In principle, yes, if you want a substituted malonic ester. But in general, one of the ester groups is decarboxylated
Q: Can you react the starting material with acid without first reacting with the alkyl halide?
A: No. The point of the other ester group is to improve the reaction by increasing the acidity of the substrate. One can alkylate an ester if the enolate is generated with LDA
Q: Have we learned how to make a malonic ester or would it be given as starting material?
A: Yes we have learned who to make malonic esters (it is the product of a Claisen condensation of ethyl acetate and diethylformate) but it is usually given as a starting material
04-05-2006
Q: on slide 248, for the beta-keto carboxylic acid and decarboxylation, you used HCl and heat, but the book just says -CO2…are they the same?
A: This is a hydrolysis of the esters, so you need aqueous acid. So it should be H3O+ or HCl and H2O plus heat. The aqueosus acid hydrolyzes the ester to a carboxylic acid which then loses CO2.
03-20-2006
Q: I was reading section 23.4 on the dehydration of aldol products and I noticed a small discrepancy between your mechanism you did in class and the book’s mechanism. In the book, it says that the carbonyl oxygen is protonated first to form the enolate ion, followed by the protonation of the beta -OH group. However, your notes show the beta -OH group protonation followed by the carbonyl protonation. If your mechanism is correct, why would the -OH group be protonated first? Wouldn’t the carbonyl oxygen be more basic?
A: I apologize; I try to be as consistent with the book as possible.
I will first explain why in this case the order doesn’t matter, as opposed to other mechanisms such as the acid-catalyzed hydrolysis of a ester where the order of events does matter. I will then explain why the book’s mechanism is better.
In the case of the acid-catalyzed dehydration, the order of enol formation and hydroxyl group protonation doesn’t matter much because these two events must both happen before dehydration can take place and one is not dependent on the other. Since both the hydroxyl protonation and enol formation are part of an equilibria, the desired hydroxyl protonated enol will be obtained some percentage of the time and this intermediate will undergo dehydration. This is opposed to other reactions such as the acid-catalyzed hydrolysis of a ester, which is also a muti-step mechanism that are each part of an equilibrium. In that case, protonation of the carbonyl group activates it towards nucleophilic addition; so the second equilibria (nucleophilic addition to the carbonyl) is dependent on the first step of the mechanism (protonation of the carbonyl group).
The mechanism in the text for the acid-catalyzed dehydration of an aldol is better than the one I drew on the board. The enol formation is acid catalyzed, so the acid is not consumed in this step. Protonation of the hydroxyl group consumes the acids until it is regenerated upon dehydration. If you protonate the hydroxyl group first (the acid is not regenerated until after dehydration), a second equivalent of acid is needed to catalyzed the enol formation. So the out of order mechanism that I drew on the board is actually sloppy in terms of keeping track of the catalyst.
Thank you for pointing that out.
03-19-2006
Q: For problem 22.27 (a), if the compound were instead meta-bromo-(3-oxobutyl)benzene, could one use an acetoacetic ester synthesis with meta-bromo-benzylbromide? (i.e., I am guessing that the aromatic bromine would not interfere with the reaction and that acetoacetic ester would react exclusively with the benzylic bromine)
A: Yes, that would be fine. The aryl bromide ewould be unreactive to the malonic acid synthesis
Q: Are there any limitations to the reagents that can be used in malonic/acetoacetic ester syntheses besides the general limitations of all SN2 reactions?
A: No, there are no general restrictions other than the limitations of the SN2 reaction
Q: Why are vinylic protons non-acidic even when the proton is on a carbon that is alpha to a carbonyl? Alkane protons which are on a carbon that is alpha to a carbonyl are acidic, and alkane protons are less acidic than alkene protons. (see problem 22.33 for clarification)
A: Normally a vinyl proton is more acidic that an alkyl proton; however, both are still very weak acids. The major effect for the increase in acidicity of protons on the alpha carbon to a carbonyl is that the anion can be stabilized through resonance into the carbonyl pi-bond. The alpha carbon of a ketone is sp3 hybridized but upon deprotonation it becomes sp2 hybridized; this puts the pair of electron in an unhybridized p-orbitals and allows the pair of electron (and charge) to be delocalized into the carbonyl pi-system. It is then analogous to the allyl anion from last semester but much more stable. If the alpha carbon is part of a vinyl group as in the example in problem 22.23, the geometry is not right for delocalization of the charge into the carbonyl group. In order to delocalize the charge into the carbonyl, the alpha carbon of 22.33 would have to become sp hybridized upon deprotonation. For what ever reason (I really don’t know), there is a very high barrier to this. There is a much larger change in geomtry for the later.
03-15-2006
Q: For Meldrum’s acid, is it the ring system that makes this compound more acid (ie. the negative charge can be distributed in the ring system as well as the carbonyl groups?)?
A: The ring is important and in a way helps to better distribute the negative charge, but it is not distributed throughout the entire ring. The ring holds both carbonyl groups co-planar which is the best conformation for delocalizing the charge into both carbonyl groups.
Q: In the HVZ reaction mechanism we did for slide 236, how does the first step
occur where PBr3 replaces OH?
A: Mechanism is very similar to the replacement of and alcohol with Br by PBr3 (see slide 98 from Ch. 17), rather than Br- participating in an SN2 reaction, and it in a nucleophilic acyl substitution reaction (similar to acid chloride formation with SOCl2). See below.
 Q: And in the second step of the aforementioned reaction, where does the H come from that protonates the O on the carbonyl?
Q: And in the second step of the aforementioned reaction, where does the H come from that protonates the O on the carbonyl?
A: It is actually the proton of the carboxylic acid. Acid bromide formation product a phosphoric acid as the by-product of PBr3
Q: Does enol formation favor C=C at the less sterically hindered site (i.e., the left most compound on slide 233)?
A: No. Since this is an equilibrium, it is governed by the thermodynamics. The enol that will be favored is the one that is thermodyanmically the most stable, So the more substituted enol is favored because the C=C bond that is most stable is the more substituted.
Q: On slide 229, when you showed us the reaction of enol to alpha-substituted product, you said that the acid catalyzed reaction would come before – would it be fine for the base catalyzed reaction to occur in place of the acid catalyzed reaction?
A: Alpha-substitution of an enol can go by either acid or base catalysis
Q: It is possible to isolate the enol intermediate, right (even though the equilibrium lies on the side of the keto form)?
A: No, the concentration of the enol is low and the rate of the equilibrium is too fast for one component to be isolated. It is generally difficult to “isolate” one component of an equilibrium because it will interconvert to the others and the equilibrium mixture will eventually be restored. If the rate of the interconversion is slow, then is is possible to isolate one component, but it will eventually re-equilibrate.
03-13-2006
Q: Page 830 of the text mentions that “aldehydes, ketones, esters, acids, and amides can be converted into enolate ions by reaction with LDA.” What about acid chlorides and anhydrides?
A: This is a good example of the basicity versus nucleophilicity. These parameters must also be measure in the contexts of the rest of the system. Acid chlorides and anhydride are generallt so reactive toward nucleophilic acyl substitution, that it would be very difficult to find a base that would rather act as a base and do an a-deprotonation over acting as a nucleophile and adding to the carbonyl. Although I can recall a very few exampled of enolate formation and anhydride, these are not compounds that enolates are generally formed from.
Q: Could you please explain the acidity order amides > nitriles > esters > ketones. Furthermore, why is the diester less acidic than the dinitrile (the mono-ester is more acidic than the mono-nitrile.
A: The amide is less acidic because the nitrogen resonance with the carbonyl reduces the ability of the carbonyl to stabilize the negative charge of the enolate. The same is true of the ester, although the resonance of esters is generally much weaker than for an amide. The acidity of nitriles also puzzles me and I always think that they should be more acidic than esters; I presume that the nitrigen is simpley not as good at stabilizing a negative charge as an oxygen.
I don’t know for sure about the acidity of 1,3-dinitriles versus 1,3-diesters but I believe that it has to do with the conformation. In order to get the maximium effect from both carbonyl groups (or both nitriles) for lowering the pKa of the a-hydrogens, they have to be co-planar. What we observe for the pka is a weighted average of all conformations present. A percentage will have both groups co-planar and a precentage will not. Since the nitrile has such low steric demands, there will be a larger percentage (than for the diester) of the conformation where both nitrile groups are co-planar and able to cooperativly stabilize the negative change.
Q: Can carboxylic acids lose their alpha proton upon reaction with LDA? They will readily lose their OH proton, acquiring a negative charge. This negative charge will reduce the acidity of the alpha proton. I guess the proper wording of the question is: is the pKa of a deprotonated carboxylic acid less than 40 (the pKa of LDA)? If so, the carboxylic acid will be doubly deprotonated to its dianionic form.
A: Your reasoning is correct. Two equiv. of LDA will give the dianion of a carboxylic acid.
Q: Does the carbonyl bond energy account for the fact that electrophiles typically react with deprotonated carbonyls as alpha-keto carbanions rather than vinylic alkoxides? Is this the same reason why the keto form is favored over the enol form in their tautomeric equilibrium?
A: Not entirely. This is a very complex questions. As it turns out, the reactivity, C versus O can be influenced by the nature of the electrophile, the counter ion of the enolate and the solvent. The Hard-Soft Acid-Base principles are often used to account for this. The O is the hard site the C is the soft site. Li counterion promotes C reacitvity because Li+ is strongly asscoaited with the O- and thereby reduces O reactivity (there is significant covalent character to the Li-O bond). Less coordinating counter ions, such as K+ promote O reactivity. Diethylether does competes less well for coordination with the counter ion than THF, so it ether promotes more C reacitivity and THF promotes more O reactivity. DMSO gives even more O reactivity. The better the leaving group, the more O reactivity is observed. So alkyl chlorides give more C-reactivity, while tosylates give more O-reacitivity.
Q: Is the haloform reaction the exact same reaction in section 22.3 of the text (p. 824)? They simply waited to discuss polyhalogenation until section 22.7? Does haloformation only apply to methyl ketones? Why can fluoroform not be generated by this reaction? What is the usefulness of a haloform? I know that deuturated chloroform is used as a solvent in 13C NMR. Would this be a useful way to produce deuturated chloroform (if the reaction was done in D2O)? Also, I know that chloroform is useful for cyclopropanation (with regards to this, why does a deprotonated chloroform molecule eject chlorine to form a carbene? This seems like rather strange chemistry..). Are chloroforms (or the other haloforms) useful for any other reactions?
A: In a sense, it is the same. For section 22.3 it is a base catayzed halogenation of an enol. The haloform reaction involves a higher concentration of hydroxide and the hydroxide is consumed when CX3 is hydrolyzed. It is mechanistcally related, but with the higher hydroxide concentration, the haloform reaction may actually go through an enolate reather than an enol. The ejection of the CX3- is unique to methyl ketones, although the base promoted a-halogenation occurs with any ketone or aldehyde.
F2 is a fluorinating agent but it is an out-of-control one that actually needs special equipment. F2 will fluorinate just about anything and eventually replace virtually and C-H bond with a C-F. There are specialized fluorination reagents, but these do not fit well with the rest of the section.
I don’t know how CDCl3 is made. The proton of chloroform is acidic, so I suspect they make CDCl3 from CHCl3 and base in D2O and exchange H for D.
The chloroform anion does decompose to dichlorocarbene, although I am not sure of the exact reactions conditions. It may be that dichlorocarbene formation is slow at room temperaturem however it is probably a side reaction. It is a strange reaction and is mechanistically similar to Cannizarro reaction. I can think of anything particulalrly usedul about haloforms other than as dihalocarbene sources.
Q: Why is it that factors that increase the carbonyl dipole moment increase its acidity and vice-versa for those which reduce the dipole moment? If the carbonyl dipole moment is increased, there is more positive charge on the carbon. Does this cause electron density on the alpha carbon to shift to the carbonyl carbon, thereby reducing the affinity of the alpha carbon for its protons?
A: Yes, that is the correct reasoning. The dipole pulls electron density from the alpha C-H bond and makes deprotonation easier. This is the inductive effect component. The resonance effect also plays a large role. Since an ester O or amide N is involved in resonance interactions with the carbonyl, the degree to which the enolate can be stabilized through resonance is decreased. This is probably the larger effect.
Q: Could you explain why benzylic and allylic groups are more reactive under SN2 conditions than alkyl groups? Also, why are vinylic groups unable to undergo SN2 reaction? The text says that backside approach is stereochemically prevented, but a model of a compound such as bromoethylene does not appear hindered.
A: We often draw the Walden inversion as a symmetrical transitions, that is the Nu-C bond is half formed while the C-X bond is half broken; this would mean that there is little if any charge build up on the carbon undergoing nucleophilic attack. This picture is not always the case. Very often C-X bond breaking is more advanced in the transition state than Nu-C bond formation and vice versa. This will leave a partial charge character on the carbon. As the C-X bond breaks in the transition state and the charge that is being generated on the carbon (either positive or negative) is stablized by the pi-bond of the vinyl or aryl group.
You could argue that backside attack of a vinyl halide is slightly more or slightly less hindered than for an alkyl halide. I don’t think that sterics are the major issue. For the Walden inversion, the transition state geometry looks like a trignonal bipyramid geometry. This requires a “reorganization” of the moleculalr orbitals and this is part of the transition state energy. The analogous Walden inversion for a vinyl halide would require a square planar transition state geometry and my feeling is that the reoranization of the MO’s would probably be significantly more costly energically.
Q: How does one control whether malonic ester is mono- or dialkylated?
A: With stoichiometry. The advantage is that the second alkylation will be slower than the first for steric reasons.
Q: The text mentions that decarboxylation can occur only for malonic acids and beta-keto acids. Can it also occur for other species with a carbonyl beta to the carboxylic acid such as beta-amido acids, beta-nitrile acids, and beta-ester acids? This nomenclature is probably incorrect. If it is unclear what I mean, I will attempt to rephrase the question.
A: Sure, anything that can stabilize the anion that is formed upon decarboxylation will work. The decarboxylation of a b-cyano acid is well known and in fact this is a variation of the malonic acid synthesis. Even a nitro group will work.
Q: Problem 22.12 on page 837 indicates that trialkylated carboxylic acids cannot be prepared. However, what if the product of the malonic ester synthesis (a dialkylated carboxylic acid) is treated with LDA to generate the enolate ion and is then reacted with an alkyl halide? Would this be an effective method of producing a trialkylated carboxylic acid?
A: Yes, that is a reasonable way to syntehszie such as a compound, although that is no longer a malonic acid synthesis.
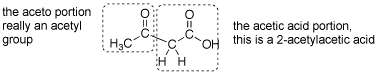
Q: Could you explain why ethyl 3-oxobutanoate is also called acetoacetic ester? Which part of the molecule is the “aceto” part and which is the “acetic”? Also, is it necessary for the ester groups to be ethoxide in both the malonic ester and acetoacetic ester syntheses? (or can they be other groups)
A: This is another reaction that is probably over 100 years old and acetoacetic acid was probably named before there was systematic nomenclature- so the name is strange. This is named as if it a 2-acetyl substotuted acetic acid. See attached gif.
Q: On page 840, the text mentions that a nonprotic solvent must be used for direct alkylation of monocarbonyl compounds. Why is this? Also, how is what is discussed on page 840 and 841 (direct alkylation) different from what was discussed on pages 834-5 when the SN2 reaction of an enolate with an alkyl halide was mentioned?
A: If you use LDA to generate the enolate, you must use a non-protic solvent because a protic solvent will be deprotonated by LDA. So if you use LDA in ethanol, what you really have is lithium ethoxide, not LDA.
Direct alkylation as described at the end of the chapter is not conceptionally different from the base catalyzed enolate formation described at the beginning of section 22.8, except for the reaction conditions are considerable different. When the enolate is generated with NaOEt in EtOH, the concentration of the enolate is related to the relative pKa’s of the base and carbonyl compound. So enolate concentration is fairly low. For direct alkylation with LDA, the enolate is generated descretely and quantitatively before the electrophile is introduced. These are much more reactive conditions. We will see the value of this more in the next Chapter.
Q: The text also mentions on page 840 that ketones, esters, and nitriles can all be directly alkylated. What about acid halides, acid anhydrides, and amides?
A: Acid chorides and anhydride can not be used to form an enolate. If the a-protons of an acid chloride is deprotonated, the Cl elimates: R2HCC(O)Cl –>R2C=C=O this is called a ketene which has unique chemistry of it own (See ketene gif). An anhydride would do the same chemistry. Enolates of amides are fine so long as there are no N-H bond invloving the amide nitrogen. So an enolate of a tertiary amide can be formed.
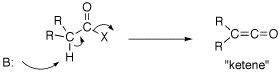
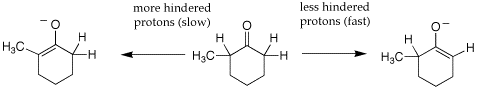
Q: The text mentions on page 841 that the major product of direct alkylation reactions generally occurs at the less-hindered (less-substituted) position. However, the enolate ion is the nucleophile and I thought that there were few restrictions on the nature of the nucleophile for SN2 reactions (the nature of the alkyl halide, by contrast, is of supreme importance). I feel that a better rationalization of the major/minor products in this instance is that the enolate ion formed at C2 is destabilized by the electron-donating methyl group relative to the enolate ion at C6. What do you think about this?
A: The text actually does not present this well. Your initial reasoning that thisis not about the sterics of the alkylation reaction is correct. It is all about the sterics of the deprotonation step. The deprotonation of an unsymmetrically substituted ketone with LDA in THF is under kinetic control since the deprotonation is essentially irreversible. So the less hindered a-proton is deprotonated fastest because it is more accessible to the base, leading to the less substituted enolate. See enolate gif. Deprotonation of the more hindered a-protons is slower but leads to a more substituted enolate which is thermodynamically favored. This is formed when the enolate is generated under reversible conditions, such as NaOEt in ethanol; this can be less selective because the ration of the more and less substitute enolate will be a function of their relative stabilisty. So this is the real value of generating enolates with LDA in THF at low temperature, you get only the kinetic enolate.
Q: The text mentions that alpha-halogenated products can be dehydrohalogenated to give alpha-beta unsaturated carbonyl compounds. Is this a good method of preparation of alpha-beta unsat. carbonyls? I thought that this reaction would give polyhalogenated products (if possible) and would therefore disallow dehydrohalogenation to form the alpha-beta carbonyl. Also, for ketones, since there are two sets of acidic hydrogens, one may have an even worse situation.
A: The polyhalogenation is more of a problem wih the base induced alpha-halogenation. These reactions are done under very basic conditions, so the enol formation is very rapid.
The reactions that we learn are fine on paper. Sometime there are disconnects in textbook; as I noted today the HVZ reaction is over 100 years old. There are more modern reagents and reaction conditions for many of these reactions that are vastly improved from the 1890’s vintage and can control which side of the carbonyl is the site for substitution and eliminated problems of multiple reactions. The alpha-bromination followed by elimination to give a a,b-unsaturated ketone is fine paper chemistry, but it is no longer the first thing that comes to mind if one really wanted to do that reaction.
The descrete formation of an enolate eliminates the issue of multiple reactions because the carbonyl is treated with an equal molar equiv. of the base to generate the enolate. Once the enolate reacts with an electrophile, there is no longer any base around to form a new enolate. So the reaction is controlled by stoichiometry.
Q: Why is the HVZ reaction needed to alpha-brominate carboxylic acids? The text states that acids, esters, and amides don’t enolize to a sufficient extent. (a) Why don’t they? (b) I thought it didn’t matter what minute amount of enol was present, simply that there iis some which can then react with an electrophile, thereby driving enolization forward. Also, can carboxylic acids be chlorinated and iodinated? Can carboxylic acid derivatives be halogenated? (it appears that only carboxylic acids can undergo the HVZ reaction)
A: This is likely to be a issue of rates. The amount of enol for a ketone or aldehyde is a small fraction of a percent. The pKa’s of acids, esters , etc, are at least 7 pKa units less acidic. It is also likely that acid catalysts doesn’t accelerated enol formation appreciably with acid derivatives to accelerate the rate of alpha halogenation enough to make the reaction useful.
I imagine that there is an anlogous set of reactions for a chlorination although I can not say that I actually remember seeing such a reaction. I do’t noe of an anlogous reaction for a-iodination.
Amides would not work under the HVZ reaction conditions for sure since they are incomparible with Br2 and PBr3. PBr3 will dehydrate the amide to a nitrile and Br2 often reacts directly with the nitrogen.
I do not recall ever seeing the alpha bromination of esters and nitrile that did not involve enolate formation.
Q: Why would one need to isolate the enolate ion (by using LDA). Could one not simply use aqueous acid to catalyze enolization. An intermediate in base-catalyzed enolization is the enolate ion. Wouldn’t this intermediate be able to undergo the desired reaction (e.g., alkylation)? What benefits are there from isolating the enolate?
A: Enolates are much more reactive than enols so they give higher yields and shorter reaction time. When one forms the enolate specifically, the problem of products derived from multiple alkylation is eliminated. We will see in the next chapter that forming descrete enolates is critical for what is know as a cross-aldol condensation. One does not isolate the enolate, it is generated discretely, then the electrophile is introduced to the reaction. This is referred to in situ.
Q: What did you mean by: “When one forms the enolate specifically, the problem of products derived from multiple alkylation is eliminated.” How does an enol give multiple alkylation products whereas an enolate ion gives but one.
This is an issue of stoichiometry. Once the enolate is formed, the base is consumed. After the enolate reacts, subsequent enolate formation can not occur because there is no longer any base present. Under the catalytic conditions, the product can be enolized again and react with the electrophile again, although the rate of the second alkylation reaction is usually slower than the first.
Q: Someone brought up in class today the question of whether enolate ions could be formed using sodium hydride. The text says sodium hydride is capable of completely effecting enolization (p. 830). Also, you mentioned that butyl lithium and sodium amide can not be used for enolization because they are too nucleophilic. How can one assess a base’s nucleophilicty. For example, how can one determine if a base is nucleophilic or non-nucleophilic. How can one determine whether a nucleophile is basic or non-basic. (as a side question, sodium amide, like LDA is also called an amide. I didn’t know if you had figured out why. I have looked (unsuccessfully) on the internet for an answer to this curiosity)
A: I thought I agreed that sodium hydride could be used, although I wasn’t sure of the pKa. It is certainly stronger than an alkoxide.
It is difficult to intuitively know what is going to be nucleophilic or basic. This is something that is learned through experience. As it turns out, many of bases such as hydroxide and amide are more basic than they are nucleophilic. However, they are nucleophilic enough to give significant side reactions. Amide would be unacceptable for formation of an ester enolate. Sodium amide can be used form ketone enolization, but it is inconvenient to prepare, relative to LDA.
I have also wondered about the nomenclature of amide but have never found a reason for it. It is unfortunate because it can cause confusion.
Exam 2 (03-01-2006)
02-27-2006
Q: I just have a quick question regarding mechanisms for tomorrow’s exam. Should we know in detail all mechanisms or should we mainly focus on the ones you actually drew and discussed on the board during class? are those the more emphasized mechanisms in the chapter – should we study those in particular?
A: Many of the mechanism are very similar and there were some that I did in class because they might help you understand (and remember) the reactions. As for the last exam, the most likely mechanisms are those that exemplify a major theme of the material.
Chapter 21
02-27-2006
Q: Do we need to know reactions or mechanisms for acid anhydrides since we did not talk about them?
A: They are exactly the same as for acid chloride. just substitute a O2CR group for
the Cl.
Q: I was told of a web site that had unique/fun ways of remembering some of the mechanisms. I thought it was too great to not send to you, so if you get a chance check out the link below:
http://www.oir.ucf.edu/chemistry/s02/frameset41.html
A: Thank you . Unique is a good way to describe it. However, can you sing the song backwards as well?
Q: Does NaBH4 not reduce any of the carboxylic acid derivatives discussed in Chapter 21? And thus it can be used to reduce other ketones and such in the presence of these carboxylic acid derivatives?
A: It does not react with esters, amides, nitriles or carboxylic acids. Acid chlorides are incompatible with the NaBH4 reaction conditions which is done in ethanol and will give the ethyl ester of the acid chloride. So that pretty much rules out carboxylic acid derivatives.
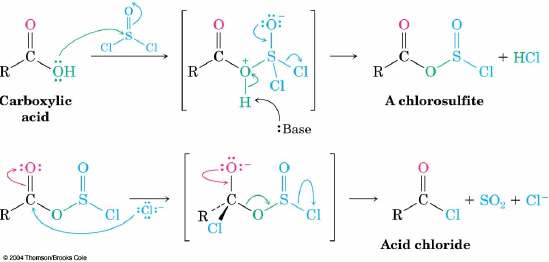
Q: On page 780 of the text book, in the mechanism of converting a carboxylic acid to an acid chloride, why does the SO2 group leave instead of Cl? I thought Cl is one of the best leaving groups because it is the conjugate base of a very strong acid.
A: I believe that you are referring to the middle intermediate (in brackets) on the second line of the mechanism. Losing chloride gets you back to the intermediate on the left. This almost certainly happens, but is an unproductive step. That step should probably be denoted as a equilibrium. Losing SO2 would be an irreversible step since it is a gas and this drives the reaction forward to give the acid chloride. Losing a neutral gas is almost always a very favorable driving force for a reaction.
Q: For problem 21.38, is the reason that there is no reactions for addition of cyclohexanol to methyl propanoate because cyclohexanol is a better leaving group than the -OCH3 of methyl propanoate
A: This is not necessarily a straightforward answer. I think the point of the problem is to stress the reactivity order of acyl derivatives; less reactive acyl derivatives can be prepared from more reactive acyl derivatives. For 21.37e, you made cyclohexyl propanoate from the acid chloride and cyclohexanol, which clearly can be done according to the reactivity order; the starting acid chloride is more reactive than the product ester, so the reaction is favored. In 21.38e you are asked if the same reaction can be done starting from methyl propanoate (an ester). Since the starting compound, methyl propanoate, is not a more reactive acyl derivative than the product, cyclohexyl propanoate (they are both esters) you were to conclude that this is not a favorable reaction- so there is no reaction. Your initial thought of looking at the relative leaving group abilities is a very good one. The pKa of methanol is ~16, but cyclohexanol (a secondary alcohol) would probably not be much different (~17), so the leaving group abilities would be roughly the same.
Q: Slide 133 shows the dipole strength of each carbonyl compound. Do these dipole strengths correspond to reactivity?
A: To a degree but no one parameter can explain everything. The prediction that ketones are more reactive than aldehydes which are more reactive than esters holds pretty well. But amides, which have one of the larger dipole are the least reactive.
Q: Are carboxylic acids are the only carbonyl compound reduced by Borane? I know that it is more selective.
A: No, aldehydes and ketones are also reduced and I suspect that an acid chloride would be reduced as well. Amides and nitriles are reduced to the amines. Ester do not react. S you note. the point is that BH3 can do selective chemistry. It reacts very quickly with carboxylic acids, so a carboxylic acid can be selectively reduced in the presence of virtually any other functional groups with BH3 without the need to use a protecting group.
02-26-2006
Q: Regarding problem 21.35 e, is it possible to get 1-butene from butanoic acid by first using LiAlH4 to form an alcahol and then form the double bond with POCl3?
A: That isn’t a great way to do it. LiAlH4 (or BH3) is correct for the reduction of butanoic acid to 1-butanol, but primary alcohols are very difficult to dehydrate to alkenes directly. So the way to go would be to convert it to a halide or tosylate followed by E2 elimination. POCl3 works for secondary and tertiary alcohols.
Chapter 20
02-28-2006
Q: I am trying to draw out the mechanism for the acid-catalyzed reaction of a nitrile to a carboxylic acid, and I’m having a hard time deciding what to do – does the N attack the H30+ and attach the whole thing, does the O from the H30+ attack the C? Then, how will I know if a water comes next to take off a H or if another water/acid attacks the C center. Is there a need to make the C a better nucleophile so it can attack water or the acid?
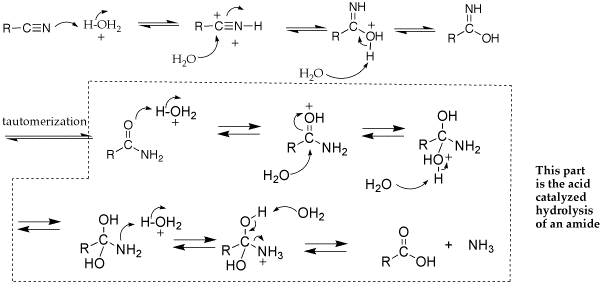
A: follow the same principles as an acid-catalyed addition to a carbonyl (i.e., acetal or enanime formation and nucleophilic acyl substitution. See mechanism below.
Q: For benzoic acids, I understand that electron withdrawing groups increase acidity by distributing negative charge, but why do they decrease reactivity and vise versa for donating groups?
A: Electron withdrawing groups makes the aromatic ring electron deficient by pulling electrons toward the EWG. This results in the aromatic ring having a partial positive charge character. This can stabilize the benzoate anion (the conjugate base of benzoic acid) through an electrostatic effect. An electron-donating groups makes the aromatic ring electron rich giving it a partial negative charge character. This destabilized the negative charge of the benzoate anion through electrostatic interactions.
02-27-2006
Q: On slide 190, where does the O come from in the final product of the reaction of nitriles with Grignard Reagents?
A: The initial product in the anion of an imine (after Grignard addition). At the end of the reaction, you add water or H3O+ to the reacion and this imine is protonated. In water, the imine will hydrolyze to the ketone plus ammonia. This is the reverse of the imine formation reaction. So the O comes from water
02-26-2006
Q: You mention on slide 185 that LiAlH4 reduces alkyl halides and epoxides. Are alkyl halides (RCH2X) reduced to RCH3 ? For epoxides, is one carbon reduced to a CH while the other becomes a C-OH? Is this reduction selective (i.e., does a certain carbon of the epoxide generally become the alcohol like we saw for acidic/basic hydrolysis of epoxides)?
A: Yes, alkyl halides are reduced to the hydrocarbon and epoxides are opened to alcohols. These reactions are likely to be SN2. For epoxides, the hydride will attack the less hindered carbon to give the more substituted alcohol.
Q: On slide 186, you mention that the carbonyl oxygen of an amide is nucleophilic and can react with electrophiles. Is this not true of all carbonyl compounds (due to the substantial dipole moment of the carbonyl functional group)?
A: Yes, but if you treat a ketone with SOCl2 nothing happens. The dipolar structure explains the reactivity of carbonyl towards most nucleophiles, which add at the C. It also explains that protons (and other strong Lewis acids) will react with a carbonyl at O, however the reactivity of the carbonyl O toward electrophiles is very modest compared to amides.
Q: What would happen if one attempted to dehydrate a secondary/tertiary amide with POCl3 or SOCl2 ? (no reaction?) Is there any way to convert these compounds to carboxylic acids?
Because there is a group or groups on the nitrogen, the dehydration can not proceed unless you break the bond between the N and the group. You end up getting a species know as a Vilsmeier salt (see attached) which is a very powerful electrophile and can be used in F-C acylation reactions. If there is nothing for this salt to react with, it will be hydrolyzed back to the original amide when you add water at the end of the reaction; the ocerall result is no reaction

Q: Why is the hydride ion the only nucleophile capable of adding twice to a nitrile? (do all other nucleophiles give the imine? is the imine stable unless hydrolyzed by aqueous acid/base to give the ketone?) I also have in my notes that only hydride ions from LiAlH4 are capable of adding twice to a nitrile. What about BH3?
A: Borane reduces nitriles to amines. I don’t know about all other nucleophiles, but since the intermediate (imine anion) from the first addition already has a negative charge, it is much harder to add a second nucleophile to give the di-anion. LiAlH4 is the only reagent reactive enough to add again. Borane is a slightly different case. Because the boron is such a powerful Lewis acid, it is strongly associated with the nitrogen- this bond has a lot of covalent character it it so the initial negative charge is largely neutralized and this allows the second hydride addition to occur.
Q: When a nitrile is reduced with DIBAL to give an imine, will the imine hydrolyze spontaneously to the aldehyde (if in aqueous solution), much like the product of Grignard addition to a nitrile spontaneously hydrolyzes to give a ketone? (does this mean that carbonyls are more stable than imines?)
A: Yes. When aqueous acid is added at the end of the DIBAH reduction or Grignard reaction, a imine that is formally derived from an aldehyde and ammonia, is obtained. This spontaneously hydrolyzes in aqueous solution to give the aldehyde and ammonia. I am not sure about stability (i.e., bond energies, etc.) but there are other considerations. The nitrogen of an imine is basic and is easily be protonated, even at neutral pH. The gives a positively charges iminium ion to which water will added very rapidily. In aqueous solution, the equilibrium is pushed to aldehyde and ammonia.
Q: If a set of atoms are conjugated, must each one of these atoms have the same hybridization?
A: Not necessarily; a C-C triple bond can be conjugated with a carbonyl, a nitrile can be in conjugation with an aromatic ring. The hydridization of the atoms must be such, that allows for an overlapping array of p-orbitals. So for an amide, the hybridization of the nitrogen is sp2 because this allows the lone pair of the nitrogen to be in a p-orbital that can conjugate with the pi-bond on the carbonyl (which are also in p-orbitals). This also true of the nitrogen of pyrrole and of an enamine (see below).
Q: You mentioned that NaBH4 is more basic than LiAlH4 and will undergo acid-base chemistry with carboxylic acids. What accounts for the basicity of NaBH4 (as opposed to lithium alumnium hydride)?
A: Any anionic species has the potential to be a base. Both are NaBH4 and LiAlH4 are basic reagents and the first mol of hydride from either will deprotonate the acid to give a carboxylate. NaBH4 is not a strong enough hydride source to react with the carboxylate further. As noted above with regard to the nitrile reduction, LiAlH4 (which is in an aprotic solvent) is reactive enough to reduce the carboxylate ion of the acid.
Q: Why are aldehydes and ketones more downfield in 13C NMR than carboxylic acids and their derivatives?
A: As we have noted the C=O bond has a large dipole moment and the carbon is deshielded as a result. For aldehydes and ketones, the alkyl groups or -H do not compensate much in terms of electron donation (which will shield the nuclei) for the large pull of the carbonyl oxygen. Carboxylic acid derivatives where the Y group can be OH, OR, NR2 or -Cl have lone pairs of electrons and can donate electron density though resonance; thus, the carbonyl carbon is not as electron deficient and consequently not as deshielded. Also, each of these group are electronegative in their own right. The individual bond dipole of the -Y group to a degree opposes the C=O bond dipole. Thus, the carbonyl carbon bond dipole is reduced and the carbon is less deshielded.
Nitrile are much more shielded for a different reason. If you remember the cryptic explanation of ring currents for aromatic rings that cause aromatic protons to be unusually deshielded, but a proton in the middle of the ring would be unusually shielded. There is a similar explaination for triples bonds. There is a ring rurrent for triple bonds that shield the carbons of nitriles and acetylenes which is why 13C and 1H resonances of these compounds are upfield compaied to alkenes.
Chapter 19
02-28-2006
Q: Ketone Hs on an alpha C don’t couple to H across C=O group, right?
A: That is correct, the alpha protons on one side of the carbonyl do NOT couple with the alpha protons on the other side of the carbonyl.
Q. Is slide 164 showing that the formation of the beta amino aldehyde/ketone is thermodynamically favored over the formation of the imine?
A: yes, conjugate addition of an amine is favored over imine formation for alpha,beta unsaturated ketones and aldehydes.
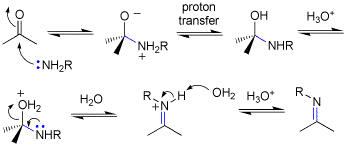
Q: The imine formation mechanism that was drawn on the board had a H on the O in the second step without any explanation of how it got there. The book mentions a proton transfer but our drawings kept the NH2R intact until a water removed one H in the third step. Did we have one too many steps with that H in our reaction?
A: See attached. The book, and I abbreviated the mechanism slightly. The prton is tranbsfered from N to O-, and this can be drawn as solvent or some conjugate base mediating the transfer as we have been drawing for other mechanisms.
Q: Slide 151 mentions that the mechanism for enamine formation includes tautomerization but I don’t see where that step comes in in the reaction shown on p. 699?
A: I mis-spoke, it is not a tautomerization. It is loss of a proton followed by rearrangment of the imine to an enamine. It is the last step, struture second to the bottom going to the bottom structure.
Q: Is the reduction of an ester to an aldehyde not a reliable reaction?
A: It is not a reliable reaction in the lab, but you can use on paper.
Q: In the reverse of the base-induced nucleophilic addition reaction to form a 1,1-diol, the second step shows the reformation of the C=O bond through the expulsion of the OH. I didn’t think that -OH was a good enough leaving group to be able to be expelled in this manner?
A: Normally it is not, but under basic conditions it there are no protons available to protonate it. Since this is cone under basic cinditions, this step generates the base catalyst and the driving force is the bond energy that is gained from re-forming the C=O.
Q: Is it CrO3 or Cr(VI) that will prepare ketones from secondary alcohols?
A: Ether PCC or CrO3/HCl will give ketones from secondary alcohols. The difference
is for primary alcohols. CrO3/HCl converts them to carboxylic acids while PCC
oxidizes them to aldehydes
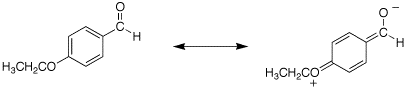
Q: The sprctra for problem 19.67c are for p-ethoxybenzaldehyde. The IR for the carbonyl is listed as 1695 cm-1. Is this correct? It does not really match that of a conjugated aldehyde which should be 1705 cm-1.
A: The carbonyl of p-ethoxybenzaldehyde really is at ~1695 cm-1. I think this is because the aromatic ring is so electron-rich due to the p-ethoxy group and an very strong contribution of the resonance form shown below.
Q: The Ylide in the Wittig reaction will only reacts with aldehyde or ketones but as far as the limitations on the reaction, are alcohols amines and carboxylic acids not tolerated in the ylide formation or the ketone or aldehyde or all? (Can you have them present anywhere?)
A: It would be a good idea not to have potentially acidic functional groups in either the ylide or reactant. Those include carboxylic acids, alcohols and amines. Esters and amides are OK but acid chlorides and anhydrides would not be.
Q: For the conjugate addition of alkyl groups via Gilman reagents, I was wondering if the Gilman’s reagent is limited to alkyl groups. I know that we said alkynyl groups don’t work in Gilman’s Reagents, but what about something like (HOCH2CH2)2CuLi ? Can functional groups such as an alcohol be added through this reaction or is it strictly alkyl groups?
A: Gilman reagents can be alkyl, vinyl or aryl.
Any basic reagent such as Grignards, Gillmans and Wittigs are going to have compatibility issues with acidic functional groups either as part of the reagent or the substrate it is reacting with. Acid base chemistry will be much faster. The key to all of these reagents is that they are negatively charged (and hence, their basic nature) nucleophiles. If they do acid base chemistry, their charge and nucleophilicity are gone. So the Gillman reagent you wrote above wouldn’t fly because of the hydroxyl group- you would not be able to generate the organilithium species. If a Wittig reagent has a ketone or aldehyde as part of the ylide, it could react with itself rather than with the substrate.
Q: I had written in class that the Wittig reaction is not compatible with acidic groups such as alcohols. Just to be sure, this means that if an alcohol were on the same molecule as the ketone reactant, the Wittig reaction wouldn’t take place?
A: Yes, the negative charge of the ylide would be re-protonated by the acidic functionality. The ylide and ketone/aldehyde substrate should be free of acidic protons. The ylide should probably not have aldehyde or ketones either since the Witting reagent would react with itself, unless this is by design.
02-27-2006
Q: The equilibrium for hydration of aldehydes and ketones favors the carbonyl compound (except with the least hindered carbonyls such as formaldehyde and acetaldehyde). Because of this, is acid or base catalyzed hydration an acceptable reaction for the synthesis of gem-diols? If not, what other methods do we know to synthesize a gem diol?
A: This is something of a model or a prototype reaction for the nucleophilic addition to carbonyls. Hydrates of ketones and aldehyde are intermediates for some reactions, i.e., Jones oxidation. Gem-diols really can not be synthesized. You put and aldehyde or ketone in water and you get an equilibrium with the hydrate. You remove the water and you get the carbonyl back.
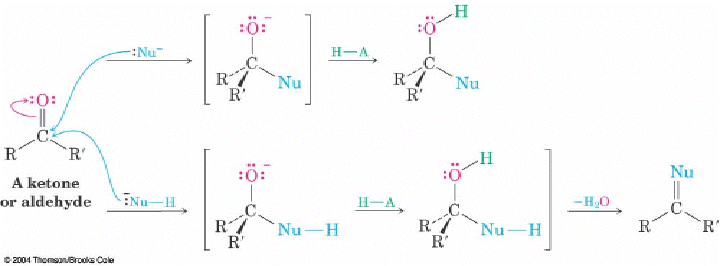
Q: On page 689 the book shows 2 different variations that a nucleophilic addition reaction can follow. What determines which variation the reaction will take? Is it the presence of a hydrogen on the nucleophile?
A: The nature of the nucleophile. The top reactivity is for hydrides and most carbon anion like Grignard reagents. The chemistry is pretty much limited to the addition step and an alcohols is usually the product. The bottom reactivity is for nucleophiles that have additions lone pairs of the electron or a proton that can be lost after the initial addition (the loss of the protons unveils a lone pair of electrons). In these cases, the lone pair can puch out the hydroxyl group (usually after protonation as H2O) to give a C=Nu. These nucelophiles have either O or N as the nucleophilic atom. For N, the C=N- is an imine and is stable or can rearrange to an enamine. Of O, the C=O-(+) is not a stable end product but is an intermediate for another nucleophilic addition (i.e. acetal formation)
02-26-2006
Q: Do you know why aldehyde/ketone hydration is more favored for less substituted molecules (e.g., formaldehyde)?
A: This probably has to so mostly with sterics.
Q: Can one control the direction of the hydration equilibrium?
A: If you remove the water, the carbonyl is produced. I don’t think that you can push the equilibrium toward hydrate.
Q: Are there any other nucleophilic addition reactions of H-Y that are favorable besides cyanohydrin formation?Do you know why does cyanohydrin formation favor the cyanohydrin?
A: Not that I can think of. Cyanohydrin formation is pretty unique.
It defies conventional wisdom because H-CN is a stronger acid that either water or an alcohol, so you would predict that the equilibrium would not favor cyanohydrin formation. However, I think the driving force must be the added energy of the new C-C bond that is being formed. Interpreting equilibrium can be tenuous because very small energy differences can significantly tip the balance of an equilibrium. An energy difference of zero means a 1:1 mixture of products and reactants. A change of just ~14 KJ/mole (which is a very small amount of energy) change the equilibrium to > 99.9 : 0.1.
Q: Are carbinolamines stable in the absence of catalyst?
A: I think you mis-spoke here. As you know, the catalyst only affects the rates of formation, not the stability of the products.
Some carbinolamines are stable and observable. Usually, there is some structural effect that holds it together, like being constrained in a ring.
Q: What is implied by the isoelectronicity of an imine with a carbonyl? That the two should exhibit very similar chemistry? Enamines are isoelectronic with enolate anions? Does this imply that the two also have similar chemistry?
A: Yes. The imine is in the same oxidation state as a carbonyl and the bonding arrangement of a C=O and C=NR are similar. So there is overlapping chemistry. A Grignard reagent will add to an imine to get an amine; they can be reduced to amines, the alpha-protons are acidic, etc. However, there is hydrolytic chemistry of imines which often competes with these reactions, which is not similar to a ketone or aldehyde
We will in the next Chapter 23 that enamines are used a enolate equivalents.
Q: Is the “alpha nitrogen” of a hydrazone (such as those generated in the Wolff-Kishner reaction) acidic (due to the neighboring imine group)? Would the acid catalyst from the previous step (hydrazone formation) have to be neutralized first? Would one just add excess base?
A: The hydrazone N-H is much more acidic than a normal N-H. There is a pH range where hydrazones can be formed. I believe that the optimal pH is just on the acidic side of neutral. But just like imines, hydrazone can be formed at basic pH, although the rate is slower (the book mentions this, but I thought that the discussion was somewhat opaque and tmight cause more questions than clarity). So the W-K reduction is most often done without a discrete acid-catalyzed formation of the hydrazone step. McMurry presents the reaction as simply hydrazine and KOH and my notes did as well. This is somewhat inconsistent with the previous acid catalyzed formation of imines, however.
Q: Are there no other functional groups that are reduced (or interfere with) the Wolff-Kishner reaction?
A: Groups that can not tolerate strongly basic conditions would not be compatible with the W-K reduction. For instance, a ester would be hydrolyzed, alkyl halides would eliminate, etc. However, these are reduction conditions that unique for ketones and aldehydes. Almost any other reducable group will not be affected by the W-K reduction, unless they were hydroxide sensitive (so an acid chloride is going to be hydrolyzed).
Q: On slide 157, a ketone is selectively reduces in the presence of an ester with NaBH4. Am I correct in saying that this would still work if the ester were a carboxylic acid instead? While the ketone would again be converted to a ketal, the acidic conditions would cause the carboxylic acid to be converted to an ester by a Fischer esterification reaction. This ester could then be reduced with LiAlH4 and the protecting group could be removed with aqueous acid. Would this be a viable way to selectively reduce a acid?
A: NaBH4 does not reduce esters, carboxylic acids, amides or nitriles. It depends on the reaction conditions, however, if the substrate on that slide were a keto-acid, I believe that you would get the acid out at the end of the reaction rather that the ester of ethylene glycol. This reaction is done in a Dean-Stark trap with a stoicheometric amount of ethylene glycol and benzene as the solvent. The Fischer esterification generally requires huge excesses of the alcohol to drive it forward. So the ketalization reaction would be faster because the ketone is more reactive. Ethylene glycol esters would be minor products of the reaction.
We noted in the Chapter 20 that borane is a method for selective reduction of a carboxylic acid. So al alternative to that shown in Slide 157 is to hydrolyzed the keto exter to a keto acid and treat with borane. This would give the keto alcohols.
Q: Why can’t tetrasubstituted alkenes be formed in the Wittig reaction (the book only goes so far as to say “steric reasons” are involved)? Is it unwise to add a disubstituted triphenylphosphine to an aldehyde to give a trisubstituted alkene? The book mentions the reaction does not work well with disubstituted CR2 groups.
A: Sterics are the issue. The mechanism of the Wittig reaction is actually a little complicated. It turns out that the initial addition of a Wittig reagent to the ketone or aldehyde is actually reversible. This is in contrast to the addition of other carbon anions such as Grignard’s, etc., where the addition is irreverible. So the collapse of the oxaphosphatane to the alkene and Ph3PO is the “committed” step. If the formation and collapse of the oxaphosphatane is slow relative to the reverse of ylide addition, there will be no reaction. For a tetrasubstitute alkene, there can be considerable steric strain because both sets of cis-groups on the alkene would be “eclipsed,” so depending on the steric requirements of these groups, collapse of am oxaphosphatane to a tetrasubstituted alkene is likely to be unfavorable. McMurry does not mention any of this an I believe that it is an unnecessary details.
Q: Are there any limitations on which molecules can be used as Wittig reagents (besides any compound containing an acidic group)?
A: It would not be a good idea to have a ketone or aldehyde as part of theWittig reagent as they can then self condense.
Q: Why is the carbonyl carbon resonance in 13C NMR so weak? Doesn’t the intensity depend on the magnitude of the dipole moment as in 1H NMR? If so, wouldn’t one expect this resonance to be strong?
A: This is really beyond the scope right now and the deeper reasons are well beyond my understanding. Almost all textbooks described NMR the way it was done 30 years ago. The NMR instruments do not work that way anymore and the physics and math required to fully understand NMR are bewildering (very advanced, at least for me).
The book very briefly mentions FT-NMR (Chapter 13.4) which is the way NMR instruments work. The dipole of the nuclei does’t play a significant role in NMR. NMR is related to the interaction of the magnetic moment of a nuclei with an external magnetic field. Not all nuclei are NMR active. In any event, the magnetization of the nuclei is perturbed by a pulse of EM energy. The nuclei react to this perturbation and this gives rise to the NMR signal. In FT- (or pulsed or time-domain) NMR this perturbation is done many times and a spectrum is collected each time. The signals are then averaged (this is called signal averaging) and converted into the spectrum. After the nuclei is perturbed, it relaxes back to it original state; this information is collected and is one spectrum; it is then perturbed again after a time delay to allow for the relaxation and this is how multiple spectra are collected and averaged. All protons relax quickly and over a fairly uniform time range, in a few milliseconds or less, so the spectra can be collected very quickly. Carbons however relax slowly relative to protons and over a large range of the relaxation rates. Most carbons relax within a second or so, but a carbonyl can take several seconds or longer to relax and this is difficult to predict. So while the data is being collected, the different rates of relaxation cause the intensities of the carbon signals to differ. The delay between pulses is often not set sufficiently long enough to allow full relaxation and this added to weak signal of the carbonyl. So carbon intensities do not reflect the relative number of nuclei giving rise to that signal and therefore can not be reliable integrated without special techniques. Because carbon is such an insensitive NMR nuclei, the intensity is sacrificed in order to obtain as many pulses as possible. While the data for a proton spectrum can usually be collected in less than a minute, carbon spectra can take hours to collect.
Q: How does one determine the oxidation state of an organic compound? For example, you mentioned that ketones and ketals are in the same oxidation state, but how would one determine this just by looking at the structures? I would imagine it involves counting the number of bonds to atoms of equal electronegativity, lesser electronegativity, and greater electronegativity and taking the sum of this along with the formal charge on the atom would give a rough indication of its oxidation state. Is this at all correct?
A: For organic compound it is really much simpler than that. A carbon has a valancy of four and we are concerned about the oxidation state of a specific carbon or functional group. A hydrocarbon is in the lowest oxidation state. The oxidation state increase when a C-H bond is replaced with a more electronegative atom or group (which is almost anything). Decreasing the hydrogen content of the molecule also counts as increasing the oxidation state. Thus, a pi-bond counts as an increase in oxidation state so an alkene would be in a higher oxidation state than a alkane, but is that same as an alcohol or alkyl halide. This has to be correct if you consider the hydration of an alkene or addition of HX to an alkene. These are not redox reactions. If you were to say the alkene is being oxidized upon hydration, there must be another product which is being reduced, which there is not. The conversion of a carbonyl to an acetal/ketal is similar to the hydration of an alkene. So the acetal/ketal carbon (which was formally the carbonyl carbon) has not changed oxidation. Slide 88 shows the oxidation the oxidations of the compounds we have discussed.
Q: Could you please explain why aromatic aldehydes are less reactive than aldehydes? Does the same reduction in reactivity apply to aromatic ketones as well?
A: Both aryl aldehydes and ketones are less reactive than aliphatic aldehydes and ketones because the aromatic ring donates electron density to the carbonyl through resonance (see page 691), This donation makes the aromatic ring electron deficient and the partial positive charge is delocalized into the ring. The reduced reactivity pertained to nucleophilic addition, since the carbonyl carbon is less electropositive in this case.
02-25-2006
Q: Do imines and enamines have synthetic usefulness? I recall you saying that imines are of crucial importance in biochemistry. Can imines or enamines be converted to nitriles or amides? Can imines be reduced to amines with lithium alumnium hydride?
A: Imines are generally used as precursors for amines. We will cover a reaction called a reductive amination in which a ketone or aldehyde is reacted with an amine under reducing conditions (such as H2, Pd/C). The amine and ketone form an iminium ion (protonated imine), which is very easily reduced to give an amine. This is one of the best ways for making substituted amines. We will see in the next chapter that enamines are the equavalent of enolates. Oximes, which are related to imines can be dehydrated to nitriles.
In biochemistry, an imine is referred to as a Schiff base. The reactions of an entire class of enzymes that are dependent on the coenzyme pyridoxal phosphate, go through a critical imine linkage between the coenzyme and the substrate. As also in the class of retinal binding to opsin, imines are often transient linkages between a receptor and a protein or enzyme and substrate.
Q: How does the chemistry of organolithiums compare to that of Grignard and Gilman reagents? The text frequently discusses the latter two but rarely mentions organolithiums.
A: I probably should just stop mentioning organolithium reagents (I guess I don’t always remember what is in the first half of the text). In general, the reactivity of a Grignard’s parallels that of an organolithium (organolithiums are actually more reactive). Gillman reagents are somewhat less reactive, but they possess unique reactivities that other organometallic reagents don’t have. The coupling reaction with an acid halide being one of them.
Q: DIBAL allows partial reduction of esters to aldehydes. Does it partially reduce any other functional groups?
A: Lactone which are cyclic esters are reduced to aldehydes. In this case the product has an appended alcohol group, which comes from the other oxygen of the lactone. Nitriles are reduced to imine by DIBAH, which hydrolyze to aldehydes. DIBAL also reduced aldehydes and ketones and is used for many types of reductions.Why are acidic groups not tolerated in the Wittig reaction?
Since the reactive form of the Wittig reagent is the ylide, the negative charge
can potentially do acid-base chemistry with acidic groups. If the ylide is re-
protonated, then the phosphonium salt results.
Q: Aryl ketones are reduced to methylene groups via hydrogen over palladium. Does this reaction also work with benzaldehyde?
A: Yes
Q: What is the mechanism for heat-induced expulsion of water from carboxylic acids to form an anhydride?
A: The mechanism I would write is one eqivalent of acid protonates a second equivalent to give a RC(=OH+)-OH and a carboxylate ion. Now draw the acyl substitution reaction as we did today for other reactions, to give the anhydride and water as the product. The reaction must be done at a temperature were the water is driven out of the reaction. If water is present, the water will simply add to the anhydride and the reverse reaction will proceed.
Q: Do anhydrides react with Grignard reagents? (it is not listed as such on page 788 of the text in Figure 21.7)
A: Yes they do. Grignard addition will give the tertiray alcohol just as for acid chlorides and esters. For the most part, anhydrides are used interchangably with acid chlorides (except for the reaction with Gillman reagents). The book notes, however, that an anhydride is actually an inefficient reagent in that two mols of the starting acid are used to make the anhydride, but if you react an anhydride with, say an amine to give an amine, only half of the anhydride reacts. The other half is lost as the carboxylate. That is OK for simple anhydrides such as acetyl anydride. However, if you wanted to make an amide from a carboxylic acid, but the carboxylic acid had to be synthesized from other starting materials, you would not want to make an anhydride out of it before reacting it with an amine. The acid chloride would be the way to go.
Q: Why is the NH2 group more nucleophilic than the OH group? Also, in the synthesis of aspirin, why does the alcohol -OH of salicylic acid rather than the carboxyl -OH react with acetic anhydride?
A: I am not sure I have a good answer for that. In general, amino groups are much more reactive than alcohols toward electrophiles. When an neutral amino group reacts with a electrophile the initial product has a positive charge on nitrogen. Nitrogen handles having a positive change very well as there are many stable ammonium salts. While oxygen can handle positive change transiently, there are really not many stable species that have a positive change on oxygen. Also, if you consider what makes for a good nucleophile (Chapter 11), good nuclephiles are the conjugate bases of weak acids (i.e., strong bases). The pka of the ammonium ion (NH4+) is about 10, while the pKa of H3O+ (a surrogate for ROH2+) is -1.7.
Q: Is the basicity of an amine comparable to the acidity of an alcohol?
A: The pKa of ammonium ions are in the 9-10 range. Most alcohols are in the 16-18 range. So there is not much acid base chemistry between an amine and a normal alcohol.
Q: Why does the book say that the conversion of acid chlorides to esters is “more general” than the conversion of acids to esters? The text claims that the Fischer esterification reaction is limited to the synthesis of methyl, ethyl, propyl, and butyl esters because of the need to use excess liquid alcohol as solvent. Why is this limiting?
The Fischer esterification is practically limited to making esters with simple alcohols because they are used a solvent. Also, as the alcohols get larger, the boiling point gets higher, so there is a practical consideration of how to get the product out of the reaction. If you wanted to make an acetate ester of an elaborate alcohol that required many steps to synthesize, you couldn’t practically use it as solvent for the Fischer esterification with acetic acid (since acetic acid would be the limiting reagent in that case). So the more efficient way to go would be to use your fancy alcohol as the limiting reagent and react it with an excess of acetyl chloide (or anhydride) in pyridine solvent.
Q: The acid chloride reduction by lithium alumnium hydride proceeds through an aldehyde intermediate before the primary alcohol is produced. Can the aldehyde be isolated with DIBAL at -78 degrees celsius? (out of curiosity, why is -78 degrees celsius often used as a reaction temperature in the book? Is this because the freezing point of CO2 (dry ice) is around this temperature and therefore is a particularly easy temperature to attain?)
A: No. With DIBAH and an ester, the intial addition of a hydride give an tetrahedral intermediate with a negative charge on oxygen. The Al of DIBAH is coordinated to this oxygen. This Al is also coodinated to the othe oxygen of the ester linkage. The bi-dentate coordination is critical at stabilizng this intermediate. if it breaks down, OR is expelled and the aldehyde is generated which will react fruther with more DIBAL to give the primary alcohol. The Al-coordinated intermediate is still in an aldehyde oxidation state and in fact can be viewed as a acetal of sorts- it does not react with nucleophiles. For an acid chloride, this intermediate does not have sufficient stability partly because chloride is such a good leaving group and partly because -Cl is not as good a ligand for Al as oxygen. So the aldehyde is generated as an intermediate in the reaction and is further reduced. When LiAlH4 is used, the nature of the Al is much different than in DIBAH, so the coordination phenomena I described above does not occur.
When you powder dry ice and add isopropyl alcohol, the temeperature of the resulting slush bath is -78° C.
Q: Can the Grignard addition to esters be paused at the ketone stage?
A: In general no. The ketone intermediate is actually more reactive toward nuleophilic addition than the starting ester. The the second equv. adds faster than the first.
Q: For the reduction of amides to amines, why does the oxygen (O-) leave instead of the amine group (as occurs in reductions of other carboxylic acid derivatives)? Is the O- a better leaving group than an amine?
A: Yes, but with LiAlH4 it is not a simple alkoxy group, it is the oxygen coordinated to the aluminium of LiAlH4. It leaves to form some aluminium oxide or something. For the reduction with borane, the oxygen leaves are a borate.
02-23-2006
Q: Could you go over problem #27 from chapter 19?
A: The geometry of the nitrogen atom is trigonal planar and it is sp2 hybridized.
The lone pair of electrons on the nitrogen are in an unhybridized p-orbital.
In the geometry shown, all carbons and the nitrogen are (or nearly are) co-planar. This geometry allows for the lone-pair of electrons on Nitrogen to beconjugated with the pi-electrons of the C=C. This is a stabilizing interaction.An important resonance structure of enamines is where there is a negative chargeon the carbon and a N=C double bond. We will see this is Chapter 22.
02-22-2006
Q: At the bottom of page 689 in the book, for the second variation > of nucleophilic addition to a ketone, why does the -OH leave. I thought -OH was a bad leaving group. Does the proton transfer occur from the nucleophile to protonate the -OH?
A: This is acyually an abbreviated mechanism. Note that the -OH actually ends up as H2O. There is a proton transfer for the -Nu-H to the -OH to give -OH2 as the leaving group. This is better shown in Fig. 19.8 (page 687) for the mechanism of imine formation
Q: In the mechanism for the Grignard reaction (p695), why does water remove the MgX?
A: The -O(-) MgX+ is more or less an ionic bond. When water or H3O+ is added at the end of the Grignard reaction the -O(-) is protonated to give the -OH, which is a covalent bond. The simple answer is that the overall bonding is energetically more favorable.
Q: I don’t understand why conjugate 1,4 addition occurs? It seems that whenever a carbonyl is present, the carbonyl carbon will always be more polarized and bear more of a partial positive charge than the Beta carbon, so why does the nucleophile attack the beta carbon to begin with?
A: This is a very complicated issue. The nature and reactivity of the nucleophiles figure into the equation as well and I think that this is beyond the scope of the course at the moment. It is based largely on experimental observations that have been formulated into a set of empirical rules rather than a theoretical analysis. It is known as hard-soft acid-base principles which takes into account the solvent conditions, the counterion and the nature of the nucleophiles and electrophiles.
To summarize the observation for conjugate (1,4-) addition versus direct (1,2-) addition:
All organometallic reagents, organolithiums, Grgnard reagents and diorganocopper reagents, will undergo direct addition to aldehydes including alpha,beta-unsaturated aldehydes.
Organolithium and Grignard reagents undergo direct addition to ketones, including alpha,beta-unsaturated ketones.
Diorganocoper reagents undergo conjugate addition to alpha,beta-unsaturated ketones, but direct addition to alpha,beta-unsaturated aldehydes.
The same thing, perhaps a little simpler: Organolithium, Grgnard reagents and diorganocopper reagents undergo direct addition of aldehydes and ketones, except for the reaction of diorganocopper reagents with alpha,beta-unsaturated ketones which results in conjugtaed addition.
Amines undergoe conjugate addition to alpha,beta- unstaurated ketones and aldehydes.
Q: Finally, in 19.16 the book goes over where different aldehydes and ketones absorb on the IR spectrum. How important is it that we know these differences and what are some general guidelines determining the absorption patterns of different ketones and aldehydes?
A: It is hard for me to answer that. The book often presents this as if you need to determine a structure only by IR. In general, no one piece of spectral data can determine the structure of a molecule conclusively. Structures are determined using a combination of spectra information. Aldehydes have a distinct chemical shift in the 1H NMR spectrum. The specific location of the C=O strtch in the IR can be very helpful in determining if the aldehyde is conjugated (to either a C=C or aromatic ring). So knowing that the typical IR stretch for an aldehyde C=O is 1730 cm-1 and that conjugation moves this number down to ~1700 is useful. The same for a ketone C=O.
I would say, that knowing the standard stretches for an aldehyde (1730), ketone (1715) and ester (1735) would be beneficial and that conjugation moves these the lower wavenumbers. For cyclic ketones, small ring sizes move the C=O stretch to higher wavenumbers. Aldehydes/ketones can be differentiated from acid/esters/amides by the 13C chemical shifts of the carbonyls.
02-17-2006
Q: On slide 159, is the Wittig reagent supposed to be the phosphonium salt or the ylide because CH2 is added, not CH3?
A: That scheme shows the reagents needed to generate the ylide. So showing the phsophonium salt plus a strong base, in this case methyllithium, indicates that the ylide is being formed. Methyl lithium deprotonates the phosphonium salt to give the ylide.
Q: On slide 160, why does the alkene form in that position? Also, why does the reagent change again? – now phosphorane
A: I will change the phosphorane to the ylide in the notes and re-post them. I think that the reactivity is better understood using the ylide form. This is a very good point.
The position of the alkene from a Wittig reaction is completely defined by the reactants and is an important feature of the Wittig. It forms between thecarbonyl carbon and the anion of the ylide. If you look at the mechanism on slide 159, the alkene forms between the green carbon (the anion of the ylide) and the blue carbon (the carbonyl carbon)
Q: Between the ylide and phosphorane shown on slide 158, does one resonance form predominate?
A: I don’t know if one resonance form contributes more than the other to the resonance hybrid. Keep in mind that that neither is the actual structure. I would say that it is a personal preference to denote the ylide or phosphorane. They are equivalent, although the ylide probably more acturatly depicts the reactive species.
Q: Regarding the cyclic acetal shown on slide 157, what is the hybridization of the C attached to both oxygens?
A: The carbon of any acetal or ketal is Sp3 hydridized. However, it is still in the same oxidation state as a ketone or aldehyde. On slide 88 (Ch. 17) there is a diagram that shows increasing oxidation state. The geminal dichloride is the same as a ketone/aldehyde and imine. The oxidation state of the acetal/ketal or hydrate is the same as the geminal dichloride. However, acetals and ketals do NOT have the same reactivity as the carbonyl
Q: On slide 155, can any strong base (NaOH or KOH) be used in the Wolff- Kishner reaction?
A: KOH is interchangeable with NaOH for the W-K reduction
Q: On slide 154, is it a base that adds to the iminium intermediate to convert it to the enamine form?
A: Yes. Water can act as a base. In this examples the acid that was used was H3O+, so H2O is the conjugate base.
Q: What does the phrase on slide 152 “imines are isoelectronic with a carbonyl” mean?
A: The C=N double bond of an imine has similar bonding properties with a C=O of a carbonyl and consequently they have similar reactivity. We will see some reactions of C=N later. Imines can be reduced (with hydride) and reacts with nucleophiles such as Grignard reagents to give amines in an analogous fashion as addition reactions to carbonyls.
Q: On slide 147, the reaction shows a ketone, but you can also hydrate an aldehyde? Are base or acid catalyzed reactions occur at the same rate when you have the choice as shown on slid 147 (with one having the hydroxide as a better nucleophile and the other having the protonated carbonyl as a better electrophile)?
A: The carbonyl in the substrate were not meant to be specific, so it can be either a ketone or aldehyde. This is the way the text denoted a general carbonyl compound. In fact, aldehydes hydrate much more readily than ketones.
Q: On slide 143, as the last reaction is written using the Gilman’s reagent, is this an SN2 reaction? If the object to make a ketone, why did our notes show production of a benzene with R-group or an alkene with R group attached?
A: No, this is not an SN2- SN2 reaction only occur when the whe the C-X has an Sp3 carbon. I don’t thing the mechanism is straigntforward for this reaction but is may be a nucleophilic acy substitution, which will be covered in Chapter 21.
The “R” denotes any organic groups and is meant to show that the reaction is general. The top of slide 143 is the Friedel-Crafts acylation which is limited to the preparation of aryl ketones. The reaction of an acid chloride with a Gilman reagent is a much more general way to make ketones, includinh a,b-unsaturated ketones and aryl ketones. Just about any acid chloride can be used, and we subsequently said that the Gilman reagent can be any have alkyl groups, vinyl groups and aryl groups (but not alkynyl). So this reaction can potentially make a ketone with a wide variety of groups on either side of the carbonyl depending on the starting acid chloride and Gilman reagent (which can be made from alkyl, vinyl and aryl halides)
Exam 1 (02-08-2006)
General Questions
02-05-2006
Q: Will our exams have a similar format as the exams from 1999 that are posted.
A: I really don’t know. As I mentioned in class, there will be some multiple choice questions, some give the reagent for the reaction problems, give the product for the reaction problems, some short synthesis problems and a spectroscopy problem.
The format is not an important issue. If a person knows his/her stuff, then the format doesn’t matter. If a person doesn’t know the material, then the format doesn’t matter. For people who are in between and believe that the format will make a difference, your time is better spent learning the material rather than worrying about the format.
Q: Did you think Roethlisberger got into the endzone for that first TD?
A: No, it looked to me that it should have been overturned on replay. Pittsburgh is really fortunate to have the lead going into halftime.
02-03-2006
Q: Are we responsible for the reactions that are from last semester?
A: I generally assume that you have master the first semester of organic chemistry. The material for this course is inherently cumulative (including things from the first semester), and you see this in some of the problems in the text, particularly those involving synthesis. So yes, particluarly those which are relevant to the current chapters (i.e., the preparation of alcohols from alkenes is relevant to Chapter 17, the SN1 and SN2 reaction was relevant to Chapters 17 and 18).
02-01-2006
Q: Will you be sending out a list the reactions and concepts that we should know and thing we don’t need to worry about or skipped?
A: I am not planning to do that. The slides should be your guide. I have followed the books closely and have organized the slides according to the corresponding section heading in the text. I have gone through the slides again and made sure of this and the revised versions are now available. Those sections that we have skipped are noted with “please read.” If there are sections from the text that I did not cover in class, then you can assume that test questions will not come from those sections. Also reactions that I or the text describes as having limited value are thing that are not important. I have also covered a few thing that were not explicitly in the book such as Frost circles and a little more detail about the effect of substituents on the acidity of phenols.
We have not gone over very many new reaction so far. There were no reactions is Chapter 15. Chapter 16 is all new and there is a summary of the reactions of Chapter 16 in the slides. These reactions are mechanistically the same. Chapter 17 has a few new reaction: the reduction of carbonyls to alcohols and the addition of Grignard reagents to carbonyls (these two reactions are fundamenatally the same). The oxidation of alcohols to carbonyls is new as is the protection of alcohols. The rest of this Chapter is a review of reactions from the first semester. There are two new reactions in Chapter 18: the epoxide opening reactions, which admittedly are not as clean and easily categorized as one would like; and the Claisen rearrangement. The rest of the chapter is really a review and applications of the SN2 and SN1 reactions.
01-27-2006
Q: On your webpage, you have posted two pages giving typical NMR and IR absorptions. Will these handouts be available during the examination, or do we need to have those values memorized?
A: Those two page as they are on the web page, will be provided with the exam (please do not bring your own tables)
Q: Is it important that we know the particular solvent to use with each reagent or reaction when carrying out a chemical step? For example, if we were performing a synthesis and used LiAlH4 without indicating that ether solvent should be used, would this be considered incorrect?
A: In general it is not necessary to indicate the solvent unless the solvent is an integral part of the reaction. For example, the dehydration of an alcohol with POCl2 requires a base which is pyridine, which also happens to be the solvent.
01-25-2006
Q: Are the organic exams scheduled for the evenings, or will they take place during class?
A: All exams will be during the normal class time (9:10-10:00 am in SC 4327).
Chapter18
02-07-2006
Q: I noticed that there were not many questions on the old exam 1 pertaining to Chapter 18. Should we expect more on epoxides and ethers than was there?
A: The book was different in 1999 and so was the class. It could be that the exam did not cover Chapter 18 or the Chapters were organized differently. I can’t remember. Don’t rely on old exams.
Q: In the synthesis of ethers, slide 114, it says that can be prepared by treating the corresponding alcohol with a strong acid – what are the acid and alcohol supposed to be in the example shown?
A: It’s ethyl alcohol and I think the acid is usally sulfuric acid.
02-05-2006
Q: Does it matter whether you use CH3-I or CH3-Br for the alkyl halide in the Williamson Ether synthesis?
A: Either is fine. In practice, CH3I is more reactive.
Q: What is the solvent for the Williamson Ether synthesis?
A: The solvent is THF, NaH (sodium hydride) is used a a base to make the alkoxide ion from the alcohol.
Q: In Chapter 18, question 18.6 a and b, is it possible to, use alkoxymercuration, start with a cyclohexanol and then add to it an alkene (rather than beginning with a cycloalkene and adding to it an alcohol)?
A: If you started with cyclohexanoland reacted that with 1-butene and Hg(OAc)2, the alkoxymercuration would add the alcohol to the more substituted carbon of the alkene. So you would get cyclohexyl (1-methyl)propyl ether, C6H11-O-CH(CH3)CH2CH3. This is the result of a Markovnikov addition. By reacting 1-butanol with cyclohexene, the alkene is symmetrical, so addition to either carbon of the double bond gives the same product.
For 18.6b, you would use benzyl alcohol (C6H5CH2OH) and ethylene (H2C=CH2).
Q: In questions 18.6 part d, is no other solvent or reagent needed to form tetrahydrofuran from the alkoxide? Does Br- just attack and remove Na+?
A: The solvent for a williamson ether synthesis is usually THF, which also happens to be the product of this reaction. That doesn’t really make much sense does it?
Na+ is the counterion for the alkoxide ( I assume you are refering to a Williamson ether synthesis). This is largely an ionic bond. The SN2 reaction diplaces bromide ion. So the product from the reaction is the ether plus NaBr.
Q: In problem 18.27 part e, what is the step in the reaction where –OCH3 and HOCH3 are added to the epoxide to open it? Have we seen this reaction before?
A: If you start with cyclohexene you can convert it to the epoxide with mCPBA. The expoxide is then opened with sodium methoxide. This is a base-catalyzed nucleophilic epoxide opening (section 18.8, page 653-4; slide 124). This gives trans-2-methoxycyclohexanol. The trans stereochemistry is because the expoxide opening is an SN2 reaction. This product is the, converted to trans-1,2-dimethoxycyclohexane by a Williamson ether synthesis (NaH and H3CI)
Q: Could you please explain the regiochemistry and stereochemistry of ether cleavage. It is very confusing as to whether selective cleavage will occur or a mixture will result.
A: This will follow directly from the SN1 vs SN2 reactions from last semester. The acid cleavage of a dialkyl ether is an SN2 reaction if the carbons involved in the ether linkage are methyl, primary or secondary (section 18.5, slide 117). The ether oxygen is protonated by acid, either HBr or HI. In the second step, the conjugate base acts as a nucleophile and attacks the ether carbons in an SN2 reaction. Becasue the steric enviornment of the electrophile is important in the SN2 reaction, the nucleophile will preferentially attck the ether carbon that is less substituted. For isopropyl ethyl ether, X- attacks the ethyl group to give isopropyl alcohol and iodoethane.
However, when one carbon of the ether is tertiary the reactions becomes SN1. When the ether oxygen of i.e.,t-butyl ethyl ether, is protonated, the unimolecular dissociation of the ether bond occurs to give the t-butyl cation and ethyl alcohol. The t-butyl cation can react with X- to give the halide, or loses a proton to give the 2-methylpropene. This happens because the unimolecular SN1 reaction can out compete the bimolecular SN2 reaction, but only when a tertiary carboncation can be produced. So the protonated ethooxy group simply acts as a leaving group
I don’t think we discussed stereochemistry with this example, although since it is an SN2 or SN1 mechanism, stereochemical consideration would follow that of the SN1 or SN2 reaction.
Q: What if there are two primary substituents on the ether? Will there be a mixture of products? What if it is ditertiary? The book indicates that such a reaction will not work. Why would this be? What if it has two secondary groups? What would the reaction be in this case? And if the molecule has 1 primary and 1 tertiary group, then the result is a mixture?
A: I would expect a mixture with some notable exceptions. For instance, if one of the ether carbons were a also benzylic or allylic, they would react preferentially to other primary alkyl carbon; those positions are exceptionally reactive toward the SN2. Conversely, A neopentyl, (H3C)3CCH2-, is exceptionally hindered even though it is primary andreacts poorly in SN2 reactions (page 351)
I would expect that a di-tertiaryether would react poorly because the ether would be hard to protonated.
In the case where the ether carbons are both secondary, I would expect a mixture, subject to excpetions noted above.
In the case of one primary and one tertiary ether carbon, I would expect the unimolecular SN1 process to be faster than a biomolecular SN2.
02-04-2006
Q: On slide 128, the CH2 hydrogens furthest to the right…would they show a separate quartet and triplet or would they show a multiplet or would they show septet?
A: No, since the molecule has symmetry, the protons on the right side of the ether oxygen are chemically equivalent to the corresponding protons on the left side. So the protons on the two CH3 groups are chemically equivalent and give a single resonance, which is split into a triplet by the neighboring CH3 group. The protons on the two CH2-groups attached to the either oxygen are chemically equivalent and give a single resoancne which is split into a triplet by the neighboring CH2 group. The protons on the two middle CH2 groups are also chemically equivalent and is split into a multiplet by the neighboring CH2 and CH3 groups.
Q: On slide 129, the two protons are doublet of doublets. Is this because each is not identical to the other and/or each is not chemically equivalent to the H attached to the other C? Why does the H(a) couple with the methyl group to give a multiplet as opposed to a triplet (begin split by the two H on the adjacent C)?
A: H(b) and H(c) are not chemically equivalent. This means that they can give rise to separate resonances and they can split each other as well as other protons. For the example on slide 128 (dipropyl ether), the protons on a CH2 do not split each other because they are chemically equivalent. Chemically equivalent protons give rise to a single resonance and do not split one another (although they can collectively split another proton (or set of protons).
Since H(b) and H(c) are not chemically equivalent, they will independently split H(a). So H(b) splits H(a) into a doublet and H(c) splits H(a) further into a doublet. H(a) is also coupled to the CH3. The CH3 further splits H(a) into a quartet. So the pattern of H(a) is a doublet of doublet of quartets, the resonance is split so much that the pattern can not be deciphered and it is called a multiplet. H(a) also splits the CH3 into a doublet. Remembers coupling (splitting) is a two way street; protons that are coupled, split each other. H(b) is split into a doublet by H(c) and further split into a doublet by H(a) to give a doublet of doublets. H(c) is split into a doublet by H(b) and further split into a double by H(a) to also give a doublet of doublets.
Q: On slide 130 for the first HNMR problem C9H11BrO, you said in class that 5 13C NMR would show that the product was monosubstituted while 4 would indicate a disubstituted pattern. In this case, we see 4 (157.5, 129.4, 120.4, and
114.4) high 13C spectra, but the compound is only monosubstituted. Why is this?
A: No. Five aromatic 13C resonances would be suspicious. A mono-substituted aromatic ring should have four aromatic 13C resonances. There would also be 4 aromatic 13C resonances for a p-disubstituted aromatic because of symmetry. These would be distinguished by the integration in the 1H spectrum.
It was the carbon spectrum of C9H10O that had five 13C resonances in the aromatic region. As it turns out, one of those was actually a vinyl carbon. The carbon was shifted downfield into the aromatic region of the spectrum because of its proximity to the aromatic ring.
Q: Can you explain the proton NMR of a monosubstituted benzene. – do they show as a multiplet or can you distinguish a series of doublets and triplets?
A: It depends on the substituents. The nature of the substituent can either shield or deshield the aromatic protons. For the 1H spectrum on slide 130 (C9H10O), you can see three distinct pattern in the for the aromatic protons that integrate to 2:2:1. This will correspond to the two ortho, two meta and one para protons. This doesn’t always happen as we have seen. Many times the sets of aromatic protons are on top on one another and the pattern is obscured. The splitting patterns of aromatic protons do not usually follow the rules of coupling that we have learned so far. This is because to this thing I mentioned called magnetic equivalence. Aromatic protons are chemically equivalent but not magnetically equivalent so the splitting pattern is often much more complicated than you would expect based on what we have learned so far. So sometimes you can distinguish coupling patterns on aromatic rings that make sense but more often they are either more complicated than you would expect or obscured by overlapping resoancnes or both.
Q: Will we always be given the number of H corresponding to each peak? –also, will we know whether it is a singlet, doublet, triplet, quartet, multiplet, etc.?
A: Yes. I will provide you with the integration, coupling pattern and coupling constants.
Q: Will hydrogens that are not chemically equivalent, but are bound to the same C couple each other? (and what about coupling with H on neighboring Cs?)
A: Non-equivalent protons on the same carbon can couple to one another and to other protons. Please note, that just because non-equivalent protons on the same carbon can couple to one another, does not mean that they will in all cases.
Q:. What pattern will you see with an o-disubstituted benzene? – will the hydrogens on the ring give two doublets and two triplets or not because they are all chemically different?
A: If the two substituents are different, then the four aromatic protons are all chemically non-equivalent. It will depend on the nature of the two substituents if you will see four distinct resonances or if they will overlap. It will also depend on the specific substance if you see a normal pattern or if it is more complicated due to the issue of magnetic non-equivalence.
It is hard to tell the positions of a disubstituted benzene based on 1H NMR. p-disubstituted give a fairly distinct pattern, but ortho and meta are difficult.
01-30-2006
Q: How can the reaction of an alcohol with PBr3 (to convert to an alkyl bromide) be done on a ring system such as cyclohexanol (see 18.30 (b)). Moreover, how can any reaction that occurs by an SN2 mechanism occur on a ring system. After reading Page 551 of McMurry, which states that carrying out an SN2 reaction a ring is a geometric impossibility because the stereochemistry of the entire ring would have to be inverted. Also, the nucleophile would have to approach the carbon atom from within the interior of the ring, and thus, steric factors make this even more unlikely. How then, is it possible to carry out an SN2 reaction on a ring system (as the solution manual did for Problem 18.30 (b)).
A: The discussion on page 551 is about aryl halides which can not occur via an SN1 or SN2 mechanism. SN1 and SN2 reactions involve an alkyl halide, meaning the the carbon attached to the halogen must be sp3. For aryl halide with o or p electron withdrawing group (usually nitro groups), what appears to be an overall nucleophilic substitution is really a two-step addition-elimination reaction and is referred to as a nucleophilic aromatic substitution reaction.
In fact, many cycloalkyl halides and particularly cyclohexyl halides, react poorly in SN2 reactions. The issues are the same, in that it is difficult (but not impossible) for the nucleophile to achieve a 180° angle from the C-X bond (backside attack) that is required for the SN2 reaction. In some cases this happen through an alternative conformations of the cyclohexane (other than a chair). However, you don’t have to worry about this.
Q: I still do not understand then, for Problem 18.30 part b how the reaction with PBr3,which is SN2, could be done. This is an SN2 reaction on cyclic molecule. It would need to achieve the 180 degree angle, as you said, and it would also need to invert the ring (correct?). This seems to be a geometric impossibility (without first breaking and then reforming the ring).
A: Because benzene is flat, the 180° approach angle for a nucleophile would require that it travel through the aromatic ring, that is through atoms. Cycloalkanes are different in that they are three dimensional, not flat like benzene. They also have other conformations through which it can react. If you make a model of cyclohexyl bromide in a chair conformation, you will see that the 180° angle from the C-Br bond can be achieved for both the equatorial and axial conformations when in an otherwise chair conformation, and the nucleophile does not need to travel through other atoms. However, the approach is not unimpeded. You might conclude from looking at models that the inversion would be more easily achieved if the C-Br bond is axial, but this is still not great. As it turns out, the SN2 displacement of a cyclohexyl bromide probably goes through an alternative, higher energy conformation called a twist boat (see page 126). In the twist boat conformation, the -CH2-CHBr-CH2 portion of the cyclohexane ring can be “flattened out”. Displacement of the halide and the “inversion” part of the SN2 mechanism will actually push the “flattened out” portion into a chair conformation. This is all very complicated. So in the end and for purposes of this class, please be assured that SN2 reaction on cyclohexanes are possible. In reality the SN2 reaction on a cyclohexyl halide is a difficult reaction and is best achieved with only the most reactive nucleophiles (cyanide ion, azide and perhaps thiolates come to mind). The major product for most other nucleophiles is usually an E2 elimination. For problem 18.30b, I would predict that 4-methylcyclohexene will be the major product
Chapter 17
04-28-2006
Q: In problem 17.40, POCl3/pyridine was used to create the double bond in part b and H3O+ was used to create the double bond in part d. Are these two reagents interchangeable?
A: No; H3O+ only worked in 17.40d because the alcohol was tertiary. This would not work for 17.40b, which is a secondary alcohol. POCl3 would work for both secondary and tertiary alcohols.
02-07-2006
Q: When determining the influence of a substituent on an alcohol, do we take into account inductive effects, resonance effects, or both?
A; If it is an alcohol there is no resoancne effect, Resonance works through pi-bonds; inductive effects work through sigma bonds. Resonance effects are potentially very important for phenols, see below.
Q: In the hydration of alkenes, does it go by anti or syn addition. Also, does oxymercuration go by syn or anti addition?
A: The only hydration of an alkene that has relative sterochemistry associated with it is hydroboration. In the case, the H- and the -OH add from the same face of the alkene (syn addition)
Q: I do not see any clear pattern between the acidity of meta and para-diubstituted phenols. Is there supposed to be any correlation? Also, I recall you mentioning that ortho-disubstituted phenols are more complex to analyze due to interactions between the two substituents. If we were given a disubstituted phenol, how would we go about determining which isomer was most acidic and which is least acidic? (on slide 86, you mention that the location of the substituent relative to the hydroxyl group of a phenol is important)
A: This has to do with the balance of the resonance effect and inductive effect. Inductive effects work from any position. In the para-position, there is also a possibility for a resonance effect since the group can interact directly with the charge. Both the powerful electron-withdrawing resonance and inductive effect, are in play for p-nitrophenol (working in the same direction). In the meta-position, there is no way for the group to directly interact with the charge of the phenoxide through resonance, so this effect is attenuated. So p-nitrophenol is more acidic than the m-isomer.
For Cl, the weak electron-donating resonance effect and strong electron-withdrawing inductive effect oppose (with respect to the phenol acidity). Since the resonance effect is lessened in the m-position, the inductive effect is stronger. Thus, m-chlorophenol is the stronger acid. In the case of methoxy, there is a strong electron-donating resonance and an electron withdrawing inductive effect. In the p-position, the resonance effect dominates and p-methoxy phenol is a slightly weaker acid than phenol itself. For the m-isomer, the inductive effect dominates and m-methoxyphenol is a slightly stronger acid than phenol itself. For a methyl group, there is only an inductive effect, so there is really little difference between the p- and m-isomers.
Ortho substituents are difficult to analyze because the groups can interact. There is steric interaction but there can also be an important electrostatic interaction (called a field effect). So we did not go into those.
For a 3,4- or 3,5-disubstituted phenol, you could predict the relative acidity only if both substituents worked in the same direction. For instance, 3,4-dinitrophenol is a stronger acid than p- or m-nitrophenol.
Q: On page 541 (figure 16.10), a phenyl group is listed as an activating substituent toward electrophilic aromatic substitution, implying that it is electron donating in nature (since all activating groups are characterized by net donation of electron density). However slide 85 indicates that a benzene ring is generally considered electron withdrawing. Could you please clarify this apparent discrepancy?
A: The resonance effect is electron donating, but the two rings must be co-planar to achieve the maximum effect. The inductive effect is electron-withdrawing. Sp2-carbon are regarded as slightly electron withdrawing as compared to an Sp3 carbon. What I meant on slide 85 is that the you would expect phenol to be more acidic than an alcohol, simply because it is on the Sp2 carbon or a benzene.
Q: I am still rather confused about the effects of the substituents in terms of Grignard reagents (slide 93). Those listed as incompatible functional groups are all slightly acidic. These are (1) incompatible with Grignard formation because they would react with themselves and (2) incompatible on the compound which will be reacted with a preformed Grignard reagent because the Grignard reagent will preferentially react with the “incompatible” group. (is this correct?) For the reactive functional groups, all except halides are sites of unsaturation (why the exception with halides?). These will all react with a preformed Grignard reagent in an intended reaction but if two or more are contained within the same compound, a mixture of products will result (is this also correct?). Now, what if these “reactive functional groups” are contained within the compound which is going to become the Grignard reagent? Will the compound react with itself or will something else happen? Also, both your slide and the book lists -SO2R (as opposed to SO3H) asbeing incompatible. What is the relation between SO2R and SO3H. I do not recall learning the former.
A: For most of the incompatible FG’s, acid-base chemistry is the problem. The Grignard reagent pulls a proton, faster than is reacts with any other function group. For reactive FG’s can react with a Grgnard reagent at a competitive rate as the desired reaction. For instance, if you were trying to add H3CMgBr to a compound that had both a ketone and an ester in it, you would get products from both types of reactions and that is regarded as bad. Amides are on the list but are actually pretty unreactive toward addition chemistry with Grignard, but are scidic enough to do the acid-base chemistry. So it not necessarily an issue of preferential reaction, but one of designing a sloppy synthesis where there are a number of potentially reactive sites.
Grignard reagents can undergo SN2 reactions with alkyl halides or tosylates like a Gilman reagent. They usually react faster with aldehydes and ketones, but this is bad form since there will be by-products and side reactions if alkyl halides were present in the substrate. Vinyl and aryl halides are fine for the most part.
Generally a unimolecular (or intramolecular) reaction is faster than bimolecular (or intermolecular) reaction. So if you try to generate a Grignard reagent from a substrate that also contains an aldehyde, you will get a cyclization reaction.
This could be a sulfone, or an SO3H (if R=OH) or a tosylate (R=OR). Although this is a gross over simplification, organometallic reagent are thought of an carbon anions. Anion can transfer an electron to other groups to give the radical. Organolithium, Gilman and Grignard reagents will do this. So these reagents can reduce a sulfone to a sulfoxide and further to a sulfide.
Q: I am also still confused about dehydration of alkenes. Both the E2 and E1 mechanisms work on tertiary and secondary alcohols. I realize that the E1 mechanism works best on tertiary alcohols and should be used almost exclusively for them, but what about the E2 mechanism using POCl3 and pyridine. Should we use this only for secondary alcohols? And we have no way of dehydrating a primary alcohol? For example, if we need to dehydrate a primary alcohol during a chemical synthesis, we should use some alternate route?
A: E1 for tertiary is fine, but E2 is OK as well. For secondary, I always tell the students to assume an E2 mechanism. POCl3, pyridine is fine. For a primary alcohol you can convert it to a tosylate or a bromide and treat with base such a t-butoxide or amide (H2N-). These tend to be more basic than nucleophilic.
Q: Can the reaction on the top of slide 102 (involving the geminal diol) be found in McMurry. If not, could you please briefly explain this, as I am unfamiliar with this reaction, namely the second two steps.
A: It is not in McMurry. There is an equilibrium between a carbonyl and its hydrate in aqueous solution. The oxidation of an aldehyde to a carboxylic acid can not be explained by the mechanism on the top of page 613 or on slide 101. The hydrate is the species that is being oxidized to the carboxylic acid. We will learn more about the addiction of nucleophiles to carbonyl in the coming chapters.
Q: Is the conversion of an alcohol to an alkyl halide followed by subsequent elimination with KOH in ethanol the best method for dehydrating a primary alcohol?
yes
Q: Are there any other ways to decrease the length of a carbon chain besides ozonolysis of alkenes and treatment of both alkenes and alkylbenzenes with KMnO4?
A: Cleavage of a vicinal diol
Q: Do we know any other carbon bond-forming reactions besides alkyne alkylation and the use of Gilman/Grignard reagents?
A; Addition of Grignard reagents, Diels-Alder reaction, Claisen rearrangment, carbene cyclopropanation, F-C reactions (off the top of my head)
They are coming up in the next couple of chapters,
02-5-2006
Q: On question #5 of the practice test, when you say optically active product, that could be either R or S, so, therefore all reagents produce some sort of optically active product, right?
A: yes
Q: For the oxidation of primary alcohols, the Jones acid is CrO3, what is Na2Cr2O7?
A: It is the same as chromic acid. If you dump it (or CrO3) in aqueous HCl you get Jones’ reagent
Q: Is it true that there is an inversion of configuration with the reaction that converts alcohols to alkyl halides?
A: With a secondary alcohol and PBr3 or SOCl2 there is inversion of stereochemistry. However, not for the reaction of a tertiary alcohol with HX.
Q: When 2-butene is produced from the elimination of 2-bromobutane, is there more cis product than trans, or are cis and trans produced in equal proportions?
A: The trans product will predominates for this product. Please note that the antiperiplanar arrangement between the H and a leaving group required for a E2 elimination could dictate that a cis double bond geometey as the product (for other examples).
Q: What is normally used for dehydration of a secondary alcohol? – H3O+ and THF or H2SO4 and H2O or POCl3 and pyridine?
A: POCl3/pyridine
Q: For the oxidation of primary alcohols, why use the chromium reagent as opposed to PCC? Do PCC and Jone’s reagent differ for secondary alcohols at all?
A: PCC and Jones reagent (chromic acid) are both chromium reagents. PCC (pyridinium chlorochromate) will oxidize a primary alcohol to the aldehyde and stop. Jones reagent (Chromic acid or CrO3/H3O+) oxidized primary alcohols to the aldehyde but then oxidized the aldehyde to the carboxylic acid. See slide 102.
PCC and Jones oxidized secondary alcohols to ketones. Jones is OK for this because a ketone can not be oxidized further. From a practical standpoint, PCC is superior because it in done in organic solvent rather than aqueous acid.
Q: When Gringard reagents react with esters, do they always add twice?
A: Yes
Q: When an ester is reduce with LiAlH4, are two primary alcohols produced?
A: An ester, R-CO2R’is reduced with LAH, the products are R-CH2OH and R’-OH ( I think this is what you mean). Normally, you are only interested in one of the alcohol products. The R’OH is not further effect for LAH fo it can be a 1°, 2°, 3° alcohol.
Q: When a carboxylic acids is reduced with LiAlH4 (LAH), is the oxygen just lost and two H’s added? Do we have a mechanism for this?
A: I think the oxygen is lost as an aluminium oxide of some sort. When the first hydride is added to a carboxylic acid, the coresponding aldehyde is produced. We did not go over the mechanism, but it is similar to the addition of a Grignard reagent to an ester (slide 92). Instead of a Grignard you have a hydride. The aldehyde is then reduced further by LAH.
A: When you reduce carboxylic acids with LiAlH(4), do you just loose the oxygen and add 2 H’s? Do we need a mechanism?
Q: I was reading through chapter 17, and I just had a question about the protection of alcohols using chlorotrimethylsilane. On page 615 of the text book, the last sentence in the first paragraph reads, “They (TMS ethers) do, however, react with aqueous acid or with fluoride ion to regenerate the alcohol.” However, during a Grignard reaction, you have to use acid (H3O+) to protonate the oxygen, but at the same time, wouldn’t that remove the TMS ether?
A: Yes, I mentioned this during class. The acid to protonate (or “work up” a Grignard reaction) the alcohol would also remove the TMS ether. I treated it as a seperate step on the slide to show that the protecting group needs to be removed.
02-04-2006
Q: The book shows that CrO3 with H3O+ can be used to oxidize secondary alcohols – is this true?
A: Yes. Both CrO3/H3O+ (Jones oxidation) and PCC will oxidized secondary alcohols to ketones. The difference comes with primary alcohols. CrO3/H3O+ oxidizeds primary alcohols to carboxylic acids. PCC oxidized primary alcohols to aldehydes.
Q: Do Grignard reagents add in a Markovnikov fashion? What is the difference between Zaitsev’s rule and markovnikov fashion?
A: Markovnikov additions additions refers to the addition across a C-C pi bond (i.e., an alkene). Grignard reagents do not react with C-C pi bonds. The react at the carbon of a carbonyl group (C=O) of aldehyde, ketones and esters to give an addition product (an alcohol)
A Markovnikov addition describes the regiochemistry of an addition across the alkene. For example, the addition of HBr an alkene for which the carbons are differentially substituted, for example, 1-butene (H3C-H2C-HC=CH2), can occur to give two products: 1-bromobutane and 2-bromobutane. Markovnikov’s rule states that the reaction proceeds to give only 2-bromobutane. This is because the intermediate carbocation that leads to 2-bromobutane product is more stable than the carbocation intermediate that leads to 1-bromobutane.
Zaitsev’s rule is used to predict the product from an elimination reaction. If we use the opposite of the above reaction, the elimination of 2-bromobutane can occur in two ways to give either 1-butene (H3C-H2C-HC=CH2) and 2-butene (cis or trans, H3C-HC=CH-CH3). Zaitsev’s rule says the elimination occurs to give the most stable alkene, which is the most substituted alkene, in this case 2-butene)
Q: Referring to problem 17.44, I thought that the most acidic one would be the least highly substituted (the phenol) but instead it ranks as second least acidic. How come?
A: It is not how many substituents but what the substituents are. Phenol is considerably more acidic than a normal alcohol because the negative charge of the phenoxide ion (the conjugate base) can be stabilized through delocalization into the aromatic ring (see Figure 17.3, page 595). Substituents on the ring can either help stabile that negative charge or destabilize that negative charge through inductive effects and/or resonance. A substituent that pushes electrons density into the aromatic ring will destabilize the negative charge (either through inductive effects or resonance) of the phenoxide ion and make it a weaker acid. An electron withdrawing group pulls electron density out the benzene ring (either through inductive effects or resonance) and further stabilizes the negative charge of the phenoxide ion and makes it a stringer acid.
So going though the substituents, p-OCH3 strongly donates electrons through resonance and destabilizes the negative charge so p-methoxyphenol is predicted to be a weaker acid than phenol itself. p-fluorophenol and p-cyanophenol have electron withdrawing groups. The fluoro group has a strong electron withdrawing inductive effect and a weak electron donating resoancne effect while the cyano (nitrile) group is a strong electron withdrawing group through both inductive and resonance effects. If you consider the activating or deactivating effect of a substituent on electrophilic aromatic substitution (Fig 16.10, page 541) you will note that the cyano group is a much stronger deactivator than a fluoro group. This reflects that the cyano group is a strong electron withdrawing group than a fluoro group. So the prediction is that p-cyanophenol is a stronger acid than p-fluorophenol and both will be stronger acids than phenol itself.
Q: With a racemic mixture, like the one produced in 17.46, would we say that while the products together are not optically active, each one is chiral? Why is it that a racemic mixture is produced in this case? – is it because the BH4 can attach from either side of the carbonyl group?
A: A racemic mixture means that there is a 50:50 mixture of enantiomers. In this case, the product from the NaBH4 reduction of 2-butanone is 2-butanol, which will be an equal amout of the (R)- and (S)-enantiomers. Carbon 2 of 2-butanol is chiral (or asymmetric or stereogenic) carbon and 2-butanol can have two non-superposable mirror isomers. Optical activity is a bulk property of a substance. A sample 2-butanol in which there is an unequal mixture of (R) and (S)- enantiomers will be optically active, although we often refer to thing being optically active as a pure enantiomer.
You are correct, the product from this reaction is chiral but since it gives a 50:50 mixture of enantiomers it is racemic. This is because the sodium borohydride adds to the carbonyl from either face.
01-27-2006
Q: Regarding problem 17.31 a, the solutions manual uses POCl3 to dehydrate 2-phenylethanol despite it being a primary alcohol. The text says that only 2° and 3° alcohols can be dehydrated using this reaction.
A: This specific reaction has the advantage in that dehydration give a double bond that is conjugates with the aromatic ring. Normally, 1° alcohols do not dehydrate well with POCl3.
Q: Regarding problem 17.31f, is there a reaction to partially reduce a carboxylic acid to an aldehyde? A subsequent chapter states that DIBAH in toluene at -77 C can be used to partially reduce esters to aldehydes. If a reaction were available that could partially reduce carboxylic acids to aldehydes, then one would be able to oxidize 2-phenylethanol to benzoic acid with KMnO4 and then partially reduce benzoic acid to benzaldehyde.
A: Diisobutyl aluminium hydride (DiBAL-H or DIBAH) is sometimes used to reduced esters (but not carboxylic acids) to aldehydes. It only works well with certain substrates. However the benzoic acid could be reduced with LAH to benzyl alcohol, then oxidized to benzaldehyde with PCC.
Q: Can PCC and the Jones reagent be used interchangeably to oxidize 2° alchols to ketones?
A: On paper, yes. Practically, PCC would be the way to go. It has the advantage of being done in organic solvent, while the solvent for Jones is 6M HCl.
Q: Regarding problem 17.33 e, if one had used acetaldehyde with 1,3-dibromopropane ( I assume this would be converted to a di-Grignard) would the product attained be 2,6-heptanedione?
A: Making the di-Grignard as you suggest would give the 2,6-heptanediol. The solutionss manual solution has methyl Grignard adding to 4-bromo-butanal. Grignard reagents will react with an alkyl halide via an SN2 reaction but will reacts with aldehydes faster. So the SM has an acceptable solution. 17.33e isn’t a great problem.
Q: I also have a question regarding reducing agents since we have seen so many thus far. Could you tell me if the following is correct? (I have paired a reducing agent with its reaction). Please correct any omissions or inaccuracies.
H2/Pd: c-c pi bonds (alkenes and alkynes reduced to alkanes)
H2/Lindlar: alkyne–>cis-alkene
H2/Li/NH3: alkyne–>trans-alkene
H2/Rh/C: benzene–>cyclohexane (does this reduce all c-c pi bonds without
reducing any c-o pi bonds?)
NaBH4: aldehydes/ketones –> alcohols
LiAlH4: aldehydes/ketones/acids/esters –> alcohols
(NaBH4 and LiAlH4 also reduce nitriles and nitro groups?)
SnCl2/H+ : nitro groups reduced
Sn or Zn (zero valent): reduce nitro groups
A: H2, Pd/C will also reduces aromatic ketones and alhehydes to the hydrocarbon.
H2, Rh/C will reduce all C-C pi-bonds to alcanes. I am not sure about ketones and aldehydes. This reaction is probably done under very high H2 pressure which will make ketones and aldehydes suceptible to reduction. Acids and esters will probably not reduce. This is not really a worthwhile reduction to commit to memory and was probably just used to illustrate how difficult it is to reduce a benzene ring.
LAH will reduced nitriles but NaBH4 will not. Both will reduced aromatic nitro groups but this is not an acceptable way to do this.
Sn(0) and Fe(0) reduction of aromatic nitro groups also require H+. This is not in the book, so we will just stick with SnCl2, H+ for this reduction.
Q: For problem 17.57, for step c I used a Gilman reagent ((CH2OH)2CuLi) with bromocyclohexane. Is this also a legal manuever?
A: Like Grignard reagents, Gilman reagents are strong bases, so having a free OH as part of the reagent would not work. The downside to the strategy you suggest is that BrCH2OH is not a stable compound (you wouldn’t necessarily know that), so you couldn’t even protect it as a TMS ether. There is an alternative protecing group that could be used, CH3OCH2OCH2Cl, which is known and would work. The CH3OCH2- can be removed with Lewis acids. I digresss and you shouldn’t worry about this.
So the better strategy using the tools we have learned is to make the Grignard reagent from bromocyclohexane and react it with formaldehyde (H2C=O). See page 602.
Q: Regarding the NMR problem 17.62, how can one tell from the given data whether the disbustituted benzene is ortho, meta, or para? I ruled out para because a para-disubstituted benzene will give two types of non-equivalent protons. And the benzene proton peaks appear far more complicated than that. However, the solutions manual claims this to be para-disubstituted. What is the best way to determine whether a disubstituted benzene is o, m, or p based on its 1-H NMR spectrum?
A: The pattern actually fits a p-disubstituted. While the pattern looks like a quartet, it is actually two sets of doublets. Unfortunately, the peaks heights for each double is not equal, which goes against what you have learned thus far about spin-spin coupling patterns. The NMR of benzenes is actually more complicated than we can go into.
13C data would have been very helpful for this problem as it would have distinquished a p-disubstituted from o- or m. Based only on 1H NMR, the two sets of doublets differentiates a p-disubstituted. For m-disubstituted, the proton between the two substituents should be a singlet- if it is split the coupling constants will be very small. For o-disubstituted, two of the protons will be doublets and two will be a doublet of doublets. However, the chemical shifts of the resonances are often not sufficiently spread out where the apttern of each aromatic proton can be distinquished; instead they falling on top of one another and obscure the patterns. The nature of the substituents determine how spread out the resonances are. So in the end, it is often difficult to determine the relative positions of disubstitued benzenes by 1H NMR alone. In such cases, an alternative answer may be acceptable if it is consistent with the given data.
Q: Would 1,3-di-tert-butyl-2-(CH2OH)benzene be an acceptable structure? (that obviously wouldn’t be acceptable nomenclature. I wasn’t sure if a CH2OH group could be called a methanol group). Perhaps the name would be: (2,6-di-tert-butylbenzene)methanol ?
A: The hard part of this problem is getting the integration correct. So going from left to right it should be 2:1:3:18.
The structure you propose (probably 2,6-di-t-butylbenzyl alcohol or (2,6-di-t-butylphenyl)methanol) would have a more complicated aromatic pattern and it would intergate to three rather than two protons. The three adjacent proton would couple to one another to give two doublet and a doublet of doublets. The fact that the aromatic protons are one singlet indicates that there are no aromatic protons on adjacent carbons and they are related by symmetry. (your proposed structure has the correct symmetry element)
The only other structure that would be acceptable (i.e., consistent with the data) is exchanging the methyl and hydroxyl groups. Since the two t-butyl groups are equivalent in the 1H NMR, they must also be related by a symmetry plane.
01-26-2006
Q: For the formation of an alkoxide ion (O of water accepts H from the alcohol), does this mean that water is a better Bronsted-Lowry base than and alcohols since an alkoxide ion is formed rather than an oxonium ion?
A: Just looking at their pKa’s, water is a strong acid than most alcohols. However, when referring to the pKa of a substance we are talking about its acid dissociation in a water solution and this is a measure of the hydronium ion concentration [H3O+]
Q: Is an alkoxide ion an example of the anion of an alcohol while an oxonium ion is the cation?
A: Alkoxide is the general name of the oxygen anion of an alcohol. An oxonium ion is a more genral name for a positively charged oxygen.
Q: When we talked about increasing the steric environment of an alcohol leading to a difficulty by water molecules to form a good sphere of solvation, why have we mentioned water molecules. (i.e. Where is the water from? or do we just mean H from other compounds?)
A: We talked about this with respect to its pKa. By definition, it means in dilute aqueous solution. So the change in the pKa of alcohols is reflective of the degree of solvant stabilization.
Q: Slide 84 states that “electron withdrawing groups make an alcohol a stronger acid by stabilizing the conjugate base (alkoxide).” Why do we mention a conjugate base and where is this coming from? I thought increasing substitution led to a less acidic alcohol?
A: When a proton dissociates from a substance H:A, the product that is left :A is refered to as the conjugate base of the acid. In the case of a alcohol RO:H dissociating to H+ and RO:-, the alkoxide is the conjugate base. The pKa (acid strength) is an expression of an equilibrium constant. For any equilibrium, factors that stablize the product side shift the equilibrium toward product. So as written, ROH <> RO- + H+, stabilizing the alkoxide makes the alcohol a stronger acid (and lowers its pKa). Things that destabilize the product side will make it weaker acid.Just as with other thing we have studied, the overall effect is actually the net result from a combination of effect, some factors work in parallel while others work in opposite directions. Simply adding a fluorine to ethanol does not increase the steric bulk of the ethanol, but certainly (F3C)3COH is bulkier than ethanol. However, the inductive effects of the flourine groups far outweigh the steric effects. In the casr of phenol, we limited our discussion to p- and m-substituents to avoid the issue of the steric effect for o-substituents. Substituents in the p- and m-positions are too far away to have a steric influence, their influence is due to inductive and resoancne effects.
Q: Regarding the resonance structures of p-nitrophenol on p. 595, the book says “the acidifying effect of an electron-withdrawing substituent is particularly noticeable with a nitro group at the ortho or para position”. Does the figure just show the nitro group at the para position and all the resonance structures associated with it? Should we be able to identify any one resonance structure that is more stable than the other? Is the goal to delocalize the negative charge into the NO2 group and is this best shown by the fifth resonance structure on p. 595?
A: Yes, Figure 17.3 (p. 595) only shows the resonance structures of p-nitrophenoxide ion. You should be able to recognize that the negative charge of the phenoxide can be delocalized into the nitro group when in the para-position. This is the important resonance structure. Please write out the resonance structures for the meta and ortho isomers and see the differences and similarities. This explaination for the affect of a substituent on the acidity of phenol has the same foundation as the the directing effects of a substituent for electrophilic aromatic substitution.
Q: For the addition of H2 to carbonyl to yield an alcohol (slide 89), one hydrogen comes from H30+ and the other hydrogen comes from H:- (a hydride). Where does the hydride come from or how is it made?
A: H2 addition (hydrogenation) to a carbonyl is not a generally used for the reduction of aldehydes and ketones to alcohols. Sodium borohydride and lithium aluminium hydride are employed for this reactions and the reactivity of these reagents can be thought of as a source of hydride, H:-
Q: In slide 89, what is the significance of the M+. Where does it come from and what does it do?
A: We often neglect counter ions particularly if they are not influenctial in a reaction; however, they exist and are necessary. When hydride is added to a carbonyl, the initial product is an alkoxide, which has a metal counterion. For a NaBH4 reduction the counterion is Na+, and Li+ for a LiAlH4 reduction. In many cases, the counterion plays a large role in the reactivity of a reagent.
01-23-2006
Q: The book mentions alchols, enols, and phenols. Do ynols exist (i.e., alcohols bonded to sp-hybridized carbon atoms)?
A: Yes, they are tautomers of a class of compounds known as ketenes R2C=C=O. Ketenes have a specialized chemistry. The ynol themselves are not usually observed.
Q: Is there a special name for protonated phenols? (or are they also called oxonium ions).
A: I am not aware of a special name for a protonated phenol. As it turns out phenols are much less basic than aliphatic alcohols and protonation of the phenol oxygen doesn’t lead to any special chemistry. For instance, phenol will not ionize to a benzene cation like other alcohols nor will it dehydrate to benzyne.
Q: I notice from the top figure on page 599 that LiAlH4 does not reduce alkenes. Is this also true for NaBH4?
A: NaBH4 is fairly specific for aldehydes and ketones (not esters, carboxylic acids, or nitriles). It does not reduce C-C pi-bonds. LiAlH4 (LAH) is much more reactive than NaBH4 and will reduced carboxylic acid derivatives but in general does not reduce C-C pi-bonds. This is not in the book and you do not need to remember this, but LAH will reduce propargyl alcohol, which has an OH on the carbon next to a C-C triple bond, to a trans allylic alcohol.
NaBH4 will reduce C=N bonds as we will see in a later chapter.
Q: On page 601, the text lists molecules which are capable of being turned into Grignard reagents. They mention primary, secondary, and tertiary alkyl, aryl, and vinylic. Are alkynl able to partake in the reaction?
A: Alkynyl halides are known (a halide on the Sp hybridized carbon of an acetylene). However, because terminal acetylenes are acidic, they can be converted into acetylide anions by treatment with strong base (Chapter 8.8). The lithium acetylide reacts as a carbon anion like Grignard reagents do. If you want MgX to be the counterion, the terminal acetylene is treated with another Grignard reagents, say methyl magneisum bromide. The methyl anion will act as a base and deprotonated the terminal acteylene and give the acetylide with MgBr+ as the counter ion.
Q: On page 603, the text does not list esters as being incompatible with Grignard formation?
A: Esters will react with Grignard reagents and should be listed. So the ester could be include as the -C(=O)R, where the R = OR’.
Q: The text does not list addition of methylmagnesiumbromide (CH3MgBr) to a benzylic ester (Ph-CO2R) as a possibility for the solution to Practice Problem 17.3 on page 603-604. I believe this should work because two equivalents of the Grignard reagent will add to an ester.
A: Addition of methylmagniesium bromide to any ester of benzoic acids (i.e., ethyl benzoate) is a perfectly good way to make 2-phenyl-2-propanol.
Q: Could 17.10 (b) be synthesized with a di-Grignard reagent (if such compounds exist)? For example, one might use a ethanoate (CH3-CO2R) with 1,5-dichloropentane in which both chlorines on the molecule are converted to Grignard reagents. This could then be used to form the cyclohexane in 17.10 (b). Or would such a reaction be non-viable? This is more a question of curiosity.
A: Yes, that bis-Grignard reagent is actually known. Although it was not used as you described, its reaction with methyl acetate would give 1-methylcyclohexanol.
Q: Is there no way to dehydrate primary alcohols? The text provides methods to dehydrate tertiary and secondary alcohols.
A: POCl3 reacts with alcohols to phosphotriesters (RO)3PO and dehydration to the alkene is only a minor product. The way to dehydrate an alcohol is to convert them to the primary bromide or tosylate and treat with a strong base (E2 reaction) such a sodium alkoxide or lithium amide- potassium tert-butoxide is the best but is not mentioned in the text.
Chapter 16
03-09-2006
Q: I was reviewing electrophilic aromatic substitution (a great spring break activity…), and looking at figure 16.15 and it has chlorine as the electrophile. How can a negatively charged species be considered an electrophile? The chlorine ion already has it’s eight valence electrons.
A: You are correct, chloride cannot (and does not) act as the electrophile. Chlorine (or more generally X2) is the electrophile. The electrophilic halogenation of benzene has overlap with the halogenation of alkenes (addition of X2 across a double bond). In that case, the pi-bond of the alkene acts as the nucleophile and attacks X2 with X- (halide ion) acting as a leaving group; for the addition to alkenes a halonium ion then results)
For electrophilic substitution of benzene, the pi-bond of benzene is significantly less nucleophilic than an alkene; using a pi-bond of benzene in a reaction will disrupt the aromaticity of the system which accounts for the low reactivity of benzenes. In order to get benzene to act as an nucleophile, the electrophile (in the case X2) has to be made more reactivity and this is done by the addition of a Lewis acid, i.e., FeCl3 for chlorination or FeBr3 for bromination. Please look at the mechanism for electrophilic bromnination on page 530 (or slides 39 and 40). The Lewis acid catalysts has a vacant oribal and can accept a pair of electrons from Br2 (which is now acting as a Lewis base). The Lewis acid-Lewis base complex (Br3Fe-Br-Br) is a much more reactive source of an electrophilic bromine because the Lewis acid complexation leads to a weaker Br-Br bond with the electrons being polarized towrd the Br that is coordinated to the Fe. Benzene then acts as a nucleophile and reacts with the Br with the partial positive charge and Br3Fe assists Br- to act as a leaving group. In the mechanism box on page 531 (Fig 3), imagine FeBr3 coordinated to the green bromine which makes the red Br carry a partial positive charge. This gives a resonance stabilized carbocation as an intermediate (middle of page 530). To complete the substitution reaction, Br- then acts as a base and deprotonated this carbocation intermediate to restore aromaticity.
02-07-2006
Q: Does benzylic bromination occur by Markovnikov addition of the Br?
A: Markovnikov addition is only for the addition across a C=C pi bond. The benzylic bromination occurs at an Sp3 hybridized carbon by a radical abstratcion of a benzylic hydrogen, then benzylic radical abstracting bromine atom from Br2. This gives the benzylic bromide and generates bromine radical
Q: On slide 75, which reaction is it that uses KMnO4 to change an aryl ketone into a carboxylic acid?
A: The KMnO4 oxidation of an alkyl side chain of ana alkylbenzene goes through aryl ketone, so an aryl ketone will also oxidized to give the benzoic acid. We did not go over that reaction
Q: This is probably not a very important question, but i am reviewing the section on reduction of aryl alkyl ketones, and it says that hydrogenation is limited to aryl alkyl ketones. Is an aryl substituent just one that is connected to an aromatic ring?
A: Aryl usually refers to an aromatic ring substituent. So an aryl alkyl ketone is an aromatic ring directly bonded to the carbonyl carbon of the ketone and an alkyl group on the other side.
As far as carbonyls go, the hydrogenation of a ketone to a CH2 is very specific to an aryl ketone. Non-aryl carbonyl will not reduce. However, C-C pi-bonds are aromatic nitro groups also reduce under these conditions.
Q: I had a quick question about a question on the practice test – number 15. In your answer key, you add the -NH2 group, then the -Cl group, and then the -NO2 group. This involves putting the -NO2 group in between the -Cl and the -NH2, instead of adding -NH2, then -NO2, and then -Cl, which doesn’t. Why can you do that? I thought it was “bad” to put substituents between other substituents.
A: If two groups are meta to one another, then it is hard to but a third group in between the two existing meta-substituents to give a 1,2,3-trisubstituted benzene. In this case, the NH2 and Cl are para, so putting the third group on isn’t a problem.
Q: If a benzene ring had two alkyl substituents, neither of which was t-butyl, and it was reacted with KMnO4, would both alkyl groups be oxidized to COOH or would a mixture of products occur?
A: Yes, both will be oxidized
Q: Am I correct in thinking that section 16.9 (benzyne) is one of the sections you just wanted us to read? I only ask because suggested problem 16.61 involves benzyne and I did not think we were responsible for that reaction.
A: yes, we skipped it
02-06-2006
Q: If a benzene ring has two alkyl substituents, neither of which are t-butyl, and it is reacted with KMnO4, would both alkyl groups be oxidized to COOH or would a mixture of products occur?
A: Both would expect both to be oxidizied.
02-05-2006
Q: In question 15 of the practice test, would it be fine if the last two steps were switched? (because NH2 can direct both additional substituents, Cl and NO2, to their respective p and o positions?)?
A: Yes.
Q: On #2 of the practice test, why does NO2 add para; can’t Br also put it at the ortho position (choice a); is it more likely to go para so as not to produce steric interference?
A: Consider the two aromatic rings. Would you consider one to be more activated (or deactivated) than the other?
Q: I am still confused about how deactivating substituents on a benzene ring affect the electrophilic aromatic substitution properties of that compound. If a benzene with a deactivating group is subjected to F-C alkylation conditions, the reaction will NOT occur? What if it is subjected to F-C acylation conditions? Does the reaction not occur or is its rate simply decreased? What about other electrophilic substitution reactions (e.g., halogenation, nitration, sulfonation)? Do these reactions not occur in the presence of a deactivating group or are they simply slowed down?
A: The FC reaction will not occur of the aromatic ring is more deactivated than a halobenzene (see fig 16.10, page 541). There is no reaction because the carbocation or oxacarbenium ion undergoes other reactions or decompositions faster than the FC reaction. So the reagent is destroyed. The reactive reagent for other electrophilic aromatic substitions reactions do not suffer this problem, so the reaction proceeds, but at a reduced rate.
Q: I presume that reaction of a tertiary-alkylbenzene will not undergo reaction with NBS and benzoyl peroxide?
A: If the benzylic carbon is tertiary, there is no reaction.
Q: Why is is that a NO2 substituent would be reduced to NH2 in the reduction of an aryl alkyl ketone?
A: An aromatic nitro group is very easily reduced. Catalytic hydrogenation reduces aromatic notro groups at a rate that is competative with an aryl ketone
Q: How do you determine whether resonance effects outweigh inductive effects or vise versa as an activator or deactivator?
A: Resonace effects will usually outweigh inductive effect. The exception will be for the halogens because they are not effect electron donors. However, the group must be able to directly interact, so the position is important. This is the case for the acidity of substituted phenols. Groups para to the hydroxy group can directly interact with the phenoxide ion through resonance, so the resonance effect is very powerful (either stabilizing or destabilizing); however, when they are on the meta position they can not directly interact with the phenoxide ion through resonance, so the inductive effect becoemes more important.
Q: If you want only a mono-substituted products from the F-C alkylation, is the best way to acylate and then reduce? When should you use alkylation over acylation?
A: Yes, the FC-acylation followed by reduction of the benzylic carbonyl group to a CH2 can be equivalent to the FC-alkylation. The real value of the F-C acylation is: 1) it has a different directing effect than a alkyl group (m vs o,p) and 2) the bigger problem with the FC-alkylation is the alkyl halide is converted to a carbocation which can undergo rearrangements (slides 49-50). The F-C acylation does not do that.
Q: Do you also get tri-substituted products in the F-C alkylationas or only mono and di?
A: The book makes a big deal about this but it really isn’t a problem. The F-C of benzene can give mono-, di- and tri-alkylated products. However, if you use benzene is large excess, only the mono-alylated product is obtained because of stoicheometry. So you can assume that you can get a mono-alkylate product from an F-C reaction.
Q: When a resonance structure places a positive charge on oxygen, is this stable because the oxygen donates electrons to the ring and increases its stability?
A: Yes, oxygen is a good electron pair donor and can handle a positive charge. This it can stabilize a positive charge through resoancne. It will destabiolizes a negative charge.
02-04-2006
Q: For a Friedel-Crafts alkylation of a phenol, does the alkyl group ever add to the C with the OH?
A: No. If that occurs, the system could not regain aromaticity. The OH of a phenol directs the FC alkylation to the ortho or para positions
Q: Is sulfonation followed by the alkai fusion reaction the fastest way to put an –OH group on a benzene ring?
A: Yes. That is the only way that we have learned.
01-22-2006
Q: For Problem 16.31, why are some rings deactivated and others aren’t? What determines if a ring is deactivated?
A: A substituted benzene is considered deactivated if the rate of electrophilic substitution is slower than for benzene. Activated is just the opposite, the rate of electrophilic substitution is faster than for benzene. The change in rate is attributed to the influence of the substituent on the pi-electrons of the aromatic ring. In general, if the net effect of the substituent is that it is electron withdrawing, the ring is deactivated. An electron withdrawing pulls electron density away from the aromatic ring making it electron deficient and consequently less nucleophilic. Electron donating groups push electron density toward the aromatic ring making it more electron rich and more nucleophilic.
The two components for determining if a substituent activates or deactivates the aromatic ring are its inductive and resonance effect. This is summarized on Table Table 16.2 and Figure 16.10. There is related discussion on these issue below.
Problem 16.31 asks you to predict where FC alkylation will occur on the given substituted benzene. FC alkylation is not compatible for all substituted benzenes and these limitations are noted in Figure 16.8. FC alkylation works for benzene, alkyl benzenes, phenols, anisoles, and halobenzenes. If does not work for other deactivated benzenes and for anilines. There is related discussion on this issue below.
(a) bromobenzene is slightly deactivated and there are no incompatible functional groups. It will undergo FC alkylation at the ortho or para-positions.
(b) m-bromophenol has a modest deactivating group and a strong activating group so overall it is activated. There are no incompatible functional groups. The directing effect for the two groups reinforce so you would expect FC alkylation to occur at the 4- and 6-positions
(c) p-chloroaniline, although aniline is an activating group it is incompatible with the FC alkylation reaction and therefore gives no reaction.
(d) 2,4 dichloronitrobenzene, this compound has three deactivating groups on it so it is strongly deactivated. A single nitro group is enough to prevent FC alkylation- no reaction
(e) 2,4-dichlorophenol. The two chloro groups are each modestly deactivating groups but the phenol is strongly activating. Overall, this is still an activated aromatic. There are no incompatible functional groups. The directing effect of the two chlorine atoms reinforce each other but are oppose the directing effect of the phenol. The phenol wins because it is the more powerful activator. FC alkylation occurs at the 6- position.
(f) benzoic acid is deactivated so this does not react in an FC alkylation
(g) p-methylsulfonic acid, the methyl group is a modest activator, the sulfonic acid group is a strong deactivator. The next effect is that the aromatic ring is deactivated and does not undergo FC alkylation.
(h) 2,5-dibromotoluene, The two bromine substituents are modestly deactivating while the methyl group is modest activator. The net effect is that the ring is probably not much different than benzene itself, perhaps very modestly activated, perhaps very modestly deactivated. It certainly is not strongly deactivated and there are no incompatible function groups. The directing effect of the two bromine oppose each other. The directing effect of the methyl group reinforces with the 5-bromo but is opposed to the 2-bromo. The methyl group is the stronger activating group and will direct the FC-alkylation to the 4-position.
01-22-2006
Q: I do not understand the mechanism whereby NH3 adds to a benzyne to form aniline. Is this similar to the addition of BH3 to an alkene to form an alcohol? Aan other strong bases be used to form a benzyne and subsequently add to the benzyne? For example, could a deprotonated terminal alkyne (acetylide anion) react with benzene to form an alkyne-substituted benzene?
A: We are skiping the section on benzyne (Ch. 16.9). The triple bond of benzyne is pretty strained. The reactivity of benzyne can be thought of as going through a zwitter ion rather than a triple bond. See the attached pict. You can draw resonance structures if benzyne in which there is a positive and negative charge on carbons instead of a triple bond. You can also draw benzyne as a di radical. so the nucleophile (ammonia) reacts with the cation to give a new zwitter ion in which an NH3+ group is now covalently bonded to the benzene and C2 is still deprotonated and has a negative charge. A proton is then transfered from the NH3 group to the carbon to give the product.
The only C-C bond forming reaction I have seen with benzyne is a Diels-Alder, which is mentioned in the book. I suspect that acetylide reacts with benzyne is a much more complex manner, but I don’t know for sure.
Q: I do not understand 16.54 (b). The last step in the mechanism, they treat the CH2Br groups with NaOH. How does result in conversion to CH2OH groups?A: This is a nucleophilic substitution reaction, most likely an SN2.
Q: On the bottom of slide 44, several condtions for the reduction of nitro groups are listed. Could you explain the two involving tin?
A: The mechanism of these reaction has not been extensively studied. Many zero valent metals are reducing agents, Fe(0) and Sn(0) are very mild ones. The mechanism is similar to a dissolving metal reaction that you learned last semester for the reduction of alkynes to trans double bonds with Li(0) in liq. NH3 and involves a series of single electron transfers from the metal to the nitro group. The oxygens of the nitro group are ultimately lost as water and the oxygens are eventually replaced by hydrogens on the nitrogen. Thus, acid is necessary. Aromatic nitro groups are among the easy functional groups to reduced
The reductions with SnCl2 or with Fe(0) or Sn(0) in acid are important because they selectively reduce aromatic nitro groups to anilines. They do not reduced ketones or C-C pi-bonds. This is opposed to H2, Pd/C which will reduce aryl ketones, and C-C pi bonds
Q: Related to the previous question below, does the limitation of F-C alkylation reactions regarding the nature of the substituent (p. 536 of McMurry, strong deactivating groups and amino groups are incompatible) also apply to the F-C acylation?
A: FC acylation of nitrobenzene does not occur. In fact nitrobenzene is often used as the solvent for the reaction. Benzenes with other strong deactivators also do not undergo FC acylation.FC acylation with anilines works poorly. If the aniline is converted to an amide, the reaction proceeds. Amides of anilines are o,p-directors and still activated, although less than aniline itself. I think is covered in Chapter 24. Phenols and anisoles are fine for the F-C acylation reaction.
So it appears as though the substituent effect for FC alkylation and acylation are the same.
Q: If the directing effects of the two groups are antagonistic, how does one decide whether two products will form or only one? This requires knowing the relative reactivity of all of the substituents, I imagine. Take for example problem 16.32 (c). The book gives two products (and identifies neither as major/minor) and rationalizes this by saying that both groups deactivate to a “similar extent” even though the relative reactivity chart provided on page 541 shows that this is actually not true (the nitro substituent is significantly more deactiving than the carboxylic acid substituent). On other problems, the book will simply give one possible product as the “correct” answer without indicating the minor product. How then, does one go about determining whether two products will form in roughly equal amounts, two products will form in different amounts (a major and a minor product), or one product will form to a significantly greater to extent than the other, thereby allowing us to neglect contributions from the other potential product?
A: This is a nuance (and perhaps a nuisance) that you are not expected to have a feel for at this stage. I agree completely with you regarding 16.32c. With the information you are given, I would expect the major product to be 3-chloro-4-nitro-benzoic acid. The opposing directing effect “rule” that the stronger activating group will dominate works best when the two groups are activating or if one is activating and the other deactivating. When both are deactivating, mixtures are much more common.
It is difficult to know for sure when to expect mixtures and what will be the major product and by how much in every case. The book gives “rules” and mechanistic rationale for the rules and in general these are pretty good predictor. So we do the best we can with these rather than trying to know some general rules and all the specific exceptions for each rules, as this would become overwhelming. However, it would be nice if the problems and answers were more self consistent or did not use a rationale that obviously isn’t well supported by the text. I can understand how this could be a source of frustration. I suggest that you work with the “rules” that are given in the text (and lecture) the best you can and you seem to be doing fine with them. If your answer differs from the answer book, it does not necessarily mean that you are wrong. You are doing the correct thing by asking when an answer in the book confuses you.
Q: How does one determine whether an electrophilic aromatic substitution reaction will or will not occur. Do strongly deactivating groups (i.e., the deactivators mentioned in the book excluding halogens) cause a reaction not to occur only for Friedels-Craft alkylation reactions? That is, do these compounds generally only decrease the rate of a reaction but in the specific case of F-C alkylation, they actually prevent the reaction from occuring?
A: This is a complex questions. The short answer is yes, electron withdrawing groups only slow down most electrophilic aromatic substitution reactions but in the case of the FC alkylations, they prevent the reaction from occurring.
The long winded answer: Most electron-withdrawing groups will only affects the rate of the reaction because nothing else can happen to the electophile or the aromatic ring. For F-C alkylation, the reactive electrophile is a carbocation, which can undergo other reactions. For instance, they can lose a proton to give alkenes. Alkenes under strongly acidic conditions polymerize. So because of the decreased rate of F-C alkylation of a deactivated benzene, the reactive electrophile (carbocation) actually undergoes other reactions fasters than electrophilic substitution. This is the case for a nitro group and a quarternary ammonium group (Ar-NR3+).
The F-C alkylation also works poorly for benzenes with some electron donating (activating) groups for other of reasons. Substituents such as carbonyl groups, hydroxyl groups or amino groups, have lone pairs of electrons and can act as a Lewis base. Aniline (amino groups) will react with AlCl3 to form a Lewis acid-Lewis base complex- a lone pair of electrons from N is donated to a vacant p-orbital of Al. The result is that the substituent now has a formal positive charge which strongly deactivates the ring toward electrophilc substitution. So a strong activating group is converted to a strong deactiving groups because of the acid-base chemistry. While aromatic ketones or esters are electron-withdrawing groups, the lone pair of electrong on the carbonyl oxygen interact readily with the Lewis acid catalysts and it is this interaction that causes aromatic carbonyl compounds (aldhydes, ketones, esters) from undergoing electrophilic substitution.
However, phenols and anisoles (methoxybenzene) are fine for F-C alkylations reactions. So here is another wrinkle. The difference between phenols or anisoles and anilines is that alcohols and ethers are weaker Lewis bases. So the complex between them and the catalyst is not as strong. In this case there will a reasonable concentration of free AlCl3 and phenol (or anisole) to react in the F-C reaction. In the case of an aniline or a carbonyl, the Lewis acid- Lewis base complex with AlCl3 is so strong that all of the aniline and AlCl3 is associated and consequently there is no reaction
The nature of the catalyst (Lewis acid) is central to all of this. The Lewis acids catalysts for electrophilic aromatic substitution are not necessarily interchangeable and there is a continuum of reactivities. FeX3 is strong enough to activate X2 for electrophilic substitution with a variety of substituted benzenes and does not have the drawbacks noted above. However, FeX3 is not a strong enough LA catalyst to generate a carbocation from an alkyl halide in most cases. So FeX3 is not a common FC alkylation catalyst.
The FC acylation suffers from many of the same drawbacks. Most oxacarbenium ions, although highly reactive, are not as fragile as alkyl carbocation. However, they react with amines and alcohols (anilines and phenols) to give amides and esters rather than the aromatic ring to give electrophilic substitution products. For F-C acylation, a stoichiometric amount of the Lewis acid catalyst is required since after electrophilic aromatic substitution the aryl ketone product will form a complex with the catalyst and prevent it from turning over.
So overall, we see that there is a narrow window of substituted benzenes for FC reactions: benzene itself, simple alkyl benzenes, halobenzenes, phenols and anisoles (or any ether of phenol). This needs to be taken into account in planning the order of reactions for a synthesis problem along with the directing effects.
01-20-2006
Q: When a substituent is o/p directing, does it electrophile add to both the o and p positions or one over the other?
A: We will assume that a single addition occurs. For ortho-para directors you usually get a mixture of ortho and para disubstituted benzenes as the product. The ratio is dependent upont the substituent and the electrophil and is not necessarily statictical, equal or easily predictable. So we will not worry about these details.
Q: Why is there a difference between o/p directing activators and deactivators since addition of the electrophile occurs at the o or p position?
A: These are two separate effects (see question below). The o,p part is the orientation of the reaction relative to the substituent. The other has to do with the rate of the reaction. An o,p-directing activator, directs electrophilic aromatic substitution to the o- and p-positions AND the reaction is faster than electrophilic substitutuion for benzene. o,p-deactivators have the same directing effect, but the rate of the reaction is slower than for benzene.
Q: Is halobenzene the same as phenol?
A: No, a halobenzene has a halogen as a substituent. Phenol has a hydroxyl group (-OH).
Q: Are the two classifications for substituents: activating/deactivating and o,m,p directing?
A: The substituent has two effects:
a) the substituent is either activating or deactivating- does the substituent make the benzene more reactive toward electrophilic aromatic substitution or less reactive (relative to benzene), and
b) orientation of further electrophilic aromatic substitution- does electrophilc aromatic substitution occur ortho, meta or para to the substituent.
Combining the two effects, substitutent are classified as either :
1) o,p-directing activators
2) m-directing deactivators, or
3) o,p-directing activators.
There are no m-directing activators (see question below and Chapter 16.6).
Q: Is a substituent with an electron withdrawing inductive effects a deactivating? (and if that substituent is not a halogen, then it is a meta-directing deactivator?)
A: The overall effect is a combination of inductive and resonances effects. All groups other than an alkyl group, will be more electronegative than C, so most substitunts will have an electron withdrawing inductive effect. Resonance effects can play a large and sometime even a dominant role the reactivity.
Q: Do substituents have either a inductive OR resonance effect?
A: All substituents have an inductive effect; not all substituents have a resonance effect. Many substituents have both an inductive and resonance effect. The combination of the inductive and resonance effects determine if the substituent is an activator or deactivator, and if it is an ortho-para or meta director
Q: Is there a relation between reactivity and orientation? i.e. do meta directors tend to be deactivating (electron withdrawing) and o/p directors tend to be activating except for the halogens which are deactivating?
A: All activators are ortho-para directors. This is usually due to an electron-donating resonance effect. The exception here is an alkyl group which has an electron donating inductive effect and no resonance effect.
All meta-directors are deactivators; however, not all deactivators are meta-directors. The halogens are othro-para directing deactivators because they have an electron donating resonance effect and a strong electron wothdrawing inductive effect.
The oxygen of phenol has an electron withdrawing inductive effect, but the lone pairs of electrons of the oxygen interact very strongly with the pi-system of the benzene ring. Therefore it is overall electron donating, activating and ortho-para directing
Q: From slide 54, what is the difference between activators and deactivators? Why can’t you have meta-activators?
A: A substituent on a benzene ring is regarded as activating when the substituted benzene is more reactive toward electrophilic aromatic substitution than benzene itself. Another way to look at it is that rate of electrophilic aromatic substitution for toluene, phenol, aniline, anisole, etc., is faster than the rate of electrophilic aromatic substitution for benzene. A deactivator is the opposite. The rate of electrophilic aromatic substitution for chlorobenzene, nitrobenzene, benzaldehyde, etc. is slower than the rate of electrophilic aromatic substitution for benzene.
The activator or deactivator designation therefore pertains to relative reactivity of the substituted benzene. Things that donate electrons make the benzene ring more electron rich and therefore more nucleophilic (faster reaction with electrophiles). Substituents that withdraw electrons make the benzene ring electron deficient, and therefore less nucleophilic because the electron are less available (slower rate of reaction with electrophiles).
The question of ortho-para versus meta directing pertains to how the substituent influences the relative stability of the intermediate carbocations from electrophilic addition. For electrophilc addition at the ortho or para positions, the positive charge of the carbocation resonances structures are spread out on the same carbons of the benzene ring (see Figures 16.12, 16.13, 16.14, and 16.15). The charge is on a different set of carbons for electrophilic addition to the meta position. The directing effect can be rationalized by analyzing the influence of the substituent on the carbocation resonance structures. We find that a carbocation resonance structure that arises from either ortho or para addition of the electrophile, places the positive charge on the carbon bearing the substituent. If the substituent is electron donating (either by inductive effects or resonance), the change can be stabilized by the substituent (see the boxed structures in Figure 16.12, 16.13 and 16.14). Thus, ortho or para addition is favored. If the substituent has an electron withdrawing resonance effect, the charge is destablized for this same carbocation resoanance structure from ortho or para addition (see the boxed structure in Figure 16.15). In this case, ortho or para addition is disfavored, leading to meta addition. While the carbocation for meta addition, is still detabilized by the elctron withdrawing group, it is less destabilzed than for ortho or para addition because none of the resonance structures place the charge next to the electron withdrawing group.
So the reason there are no meta directing activators is because the carbocation intermediate from ortho or para addition will always be more stable for an activating group.
Q: When you say “electron withdrawing substituents” on slide 47, do you mean a substituent with very electronegative atoms like O, S, and N?
A: Those are electron withdrawing through inductive effects, which is one part of the overall effect. You also have to consider resonance effects, which can sometimes outweigh the inductive effect. This is the case for the -OH of phenol, -OCH3 of anisole, -NH2 for aniline, etc. These groups have a more electronegative atom (than C) attached to the benzene ring and therefore have an electron withdrawing inductive effect. However, these groups have a very strong electron donating resonance effect which outweighs the inductive effect, and therefore the overall effect for these substituents is that they are strong electron donating groups.
Q: You said that the alkyl halide does not act as the electrophile but on slide 46 you have termed it that.
A: Alkyl halides are generally regarded as electrophiles, however they are not reactive enough to react with a benzene ring, which is a very weak nucleophile. So for the FC alkylation the actual electrophilic species is the alkyl carbocation. When we write out the reaction and identify the participants, we would generally call the alkyl halide the electrophile since it directly gives rise to the reactive electrophilic species.
Q: On slide 44, do all reagants/catalysts from (a) or all from (b) reduce aromatic nitro groups to the aniline? Is one combination preferred over the other? What is unique about these reagents ?
A: For the reduction of nitro group, each line has the complete set of reagents needed to carry out the reduction to the aniline. So, one set of conditions in SnCl2, H3O+ followed by hydroxide. A second set of reaction conditions is Fe(0) in acid, etc. SnCl2 is useful because it will only reduce aromatic nitro groups to anilines; C=C double bonds, etc are not reduced. H2, Pd/C on the other hand, will reduce aromatic notro groups, carbon-carbon pi-bonds, aromatic carbonyl groups with no functional groups selectivity. (see Chapter 16.11 and slides 70-72)
Q: From slide 43, would an example of a base that removes the proton from the carbocation to give the electrophilic substitution product be Cl- (left over from Cl2)?
A: Yes. Electophilic addition by X2 and FeX3 to benzene gives a resonances stabilized carbocation intermediate. The counter ion is FeX4- (anion), which is in equilibrium with FeX3 and X-. The X- acts as a base and removes a proton from the carbocation, thus restoring aromaticity and giving the substitution product. So in all cases, HX is a byproduct from electrophilic aromatic substitution. In the case of electrophilic iodination, the base is from the Cu(II) ligands.
Q: Is there an actual 3D complex made between the Br2 and the FeBr3 that allows Br to be attacked by the benzene ring?
A: Yes. There is a physical association between the FeBr3 and the Br2. This polarizes the Br-Br bond so that the Br is more electrophilic.
Chapter 15
02-07-2006
Q: On slide 75, which reaction is it that uses KMnO4 to change an aryl ketone into a carboxylic acid?
A: The KMnO4 oxidation of an alkyl side chain of ana alkylbenzene goes through aryl ketone, so an aryl ketone will also oxidized to give the benzoic acid. We did not go over that reaction
Q: If we add electrons from the negative charge as in the case of the cyclopropenyl anion for a total of 4 pi electrons, why do we not subtract the total number of electrons in the cation of clorocyclopropene? Also, why is it that only the cation of chlorocyclopropene and not chlorocyclopropene itself is described as being aromatic?
A: We do. The cycloprpenyl anion has two electron from the double bond and two from the negative charge. It has four pi-electrons and is antiaromatic. Chlorocyclopropene is the precursor to the cycloprpenyl cation. The chloro leaves with it pair of electron just like for the mechanism of an SN1 reaction. That leaves a vacent p-orbital. The cycloprpenyl cation has 2-pi electrons and is aromatic. (4N+2=2, N=0)
Chlorocyclopropene and cyclopropene do not fullfil the requirements for aromaticity or antiaromaticity in that they are not fully conjugated aroung the ring. On carbon is sp3 hybridized.
02-05-2006
Q: Is cyclooctatetraene NOT anti-aromatic? On slide 18 of the Chapter 15 slides, you mention that anti-aromatic compounds must be flat? So does cyclooctatetraene have any special classification?
A: If it is not flat then it is non-aromatic. One of the criteria for aromaticity or anti-aromaticity is that it be a planar, cyclic and conjugated. Cyclooctatetraiene adopts a nonplanar conformation to avoid being anti-aromatic.
Q: For Frost circles, must the cyclic conjugated molecule which is inscribed in the circle be flat? Can the MOs of cyclooctatetraene be determined using Frost circles?
A: Yes, one of the criteria for the analysis is that the compound be planar. The frost circle gives you an approximation of the relative energies of the MO’s. This is very useful for aromaticity analysis. It does not give the phases of the orbitals. You can now see why it is anti-aromatic.
Q: Can anti-aromatic compounds be prepared or are they more of a theoretical entitity?
A: The cyclopropenyl anion has not been directly observed. If you look at the Frost circles, you will not that the two half filled orbitals are actually antibonding orbitals (slide 21). This makes the cyclopropenyl anion exceptionally unstable. Cyclobutadiene has been observed in a frozen matrix. It is generated in the gas phase then shot into either liquid nitrogen (77 K) or liquid helium (4 K) temperature as a dilute solution in nitrogen gas or argon gas. So it is observed in a frozen matrix if nitrogen or argon. Many antiaromatic compounds have been observed.
Q: In the practice test on question 12a, I thought there would be three conjugated p-orbitals, as there are three sets of pi bonds. Is it necessary to put the shape of the compound in the Frost Circle to see the number of conjugated p-orbitals – it is not affected by number of electrons, then?
A: Each pi-bond consists of two p-orbitals. There is also a vacant p-orbital of the cationic carbon.
The Frost circle gives you the approximate relative energies of the molecular orbitals. This diagram is necessary to show why things are aromatic or antiaromatic (slide 19)
Q: In question #8 from the practice test, to be anti-aromatic, the compound must have 4n pi-electrons. Therefore, what is the difference between compounds b) and d)? Also, when totaling the number of electrons, you don’t count those influence by the compound being an ion – a cation (one less electron) or an anion (onemore electron), right?
A: The cation has two less and the anion has two more. loss or gain of one electron would give a radical which are charge neutral
Q: Please explain #7 from the practice test. How does this compound satisfy Huckel’s 4n + 2 rule?
A: Please refer to section 15.7 (page 510) and inpartcular the answer for Practice problem 15.1 (p 511-2) The oxygen can contribute a lone pair (2 electrons) making it a six pi-electron system.
Q: Since some aromatic H’s overlap in H NMR spectra, how can you determine the number of different types of hydrogens? Is there a method that can compensate?
A: The integration can tell you the number of aromatic protons and thus gives you the number of substituents. The 13C spectra can tell you about symmetry. I have answer related questions, so I am going to refer you other answers on the class Q&A page.
Q: Nitrogen contributes one pi electron to pyridine and two to pyrrole, is this determined by how many are needed for aromaticity? Why is it?
A: The nitrogen of pyridine contribute one electron to the sextet because it is formally invovled in a C-N double (pi-) bond. In pyrrole, the there are four electron from the two double bonds. The nitogen lone pair makes it an aromatic system (it is electronically similar to the cyclopentadienyl anion)
02-04-2006
Q: I was doing the suggested preactice problem 15.44 and it required knowing the IR absorptions indicating ortho-, meta-, and para- distubstituted benzenes. This was not on the provided IR absorption list. Will we be responsible for these numbers for the test?
A: As I noted in class, I do not believe that these are reliable since they are in the fingerprint region of the IR, which is difficult to interpret. We have discussed this issue regarding 1H and 13C NMR. p-disusbtitued benzenes give a reliable diagnostic pattern in the 1H NMR and since it has a plane of symmetry, will have four 13C resonances in the aromatic region. It is hard to definitively determine ortho and meta disubstituted benzenes.
The answer of a spectral problems needs to be consistent with the available data. If ortho and meta can not be determined from the data, then either answer will be fine.
So to answer you question directly, the NMR and IR tables will be provided as they currently are. You can memorize the the IR absorptions if you like, but I do not believe that is a good use of your time.
01-21-2006
Q: Does the sprcture of 15.46 have a fused ring? How are we suppose to realize that from the information given?
A: I don’t think 15.46 has a fused ring. It actually has two monosubstituted aromatic rings.
The molecular formula tells you that there are 9 degrees of unsaturation, which is unusually high. A benzene ring accounts for four; it is a reasonable assumption that this molecule has a benzene ring because of the high degree of unsaturation and the resonance at ~7.4 ppm, which is diagnostic for a phenyl substituent.
The resonance at ~5.5 ppm is in the vinyl region indicating a C=C double bond , which would account for another degree of unsaturation.
The relative integration of the resonances at 7.4 and 5.5 ppm is approximately 5:1 (I measured the integrals with a ruler and came up with ~28 mm to 5 mm). The formula tells you that there are 12 protons, so the actual ratio is 10H’s and 2H’s. So this is beginning to look as though there are two monosubstituted phenyl groups. Two phenyl groups and a C=C double bound adds up to 9 degrees of unsaturation. Two mono-substituted phenyl groups (12 C’s and 10 H’s) and a C=C double bond (2 C’s and 2H’s) is correct for the molecular formula (C14H12). Piecing this information together, the possible structures are 1,1-diphenylethylene and (E)- or (Z)-1,2-diphenylethylene. I would regard all of these structures as consistent with the data that was given. The actual answer is 1,1-diphenylethylene. The vinyl carbon for (E)- and (Z)-1,2-diphenylethylene has both the proton and a phenyl substitutent on it. Since the phenyl group is on the same carbon as the vinyl proton, the resonance of the vinyl proton is moved downfield. This is a fine point that you are not expected to know.
Q: Will the actural NMR and IR spectra be givien on the exams as on the slides?
A: I may give the actual spectra but it is more likely that I will give the data in a tabular form. You will get the chemical shift, integration, splitting pattern and coupling constants. You will not have to measure any of these thing.
01-19-2006
Q: For the spectral problem15.45, how do you know the relative psoition the Br for part a and CH3 for part c.
A: On page 515, the books notes that p-dissubstituted benzens have an IR absorbance between 810-840 wavenumbers. 15.45a and c have an aborsorbance at 820 wavenumbers. I believe that IR absorbances in that region are hard to interpret. The pattern of the aromatic protons in the NMR is diagnostic for a p-disubstituted benzene. A p-disubstituted benzene has a plain of symmetry, so that H2 is equivalent to H6, and H3 is equivalent to H5. As a result there are two resonances in the NMR, each integrating to two protons. Each resonance is split into a pattern that approximates a doublet. The pattern of the aromatic protons for 45a and c are typical for a p-disubstituted benzene.
01-17-2006
Q: The book isn’t really clear on how to determine if a polycyclic compound is aromatic. It says that the Huckel rule is only for monocyclic compounds, but goes on to say that since napthalene has 10 pi electrons which is a Huckel number, then it will have aromaticity. Is looking for Huckel numbers of pi electrons a useful way to determine aromaticity for polycyclic compounds?
A: The book is vague because it is not an easy question to address. Huckel’s rules work very well for monocyclic, conjugated systems. The rules are not as reliable when applied to polycyclic aromatics, so any conclusions from such an analysis are suspect. So there is really no simple way to look at a polycyclic aromatic compound and determine that it is truly aromatic, i.e., has a special stabilization attributed to the cyclic conjugated system. However, if you can draw a cyclic structure that is completely conjugated and it has 4n+2 pi-electrons, it is likely that it will have some aromatic stabilization. As it turns out, polycyclic aromatics compounds are much more reactive than benzene. The central ring of anthracene (see problem 15.25 for the structure) for instance, will actually act as a diene and undergo a Diels-Alder reaction with certain dienophiles. One double bond of the central ring of phenanthrene (see problem 15.26 for structure) is also much more reactive than you would expect for an typical aromatic compound. So while many of the polycyclic aromatic compounds are aromatic, they do not have the same degree of aromatic stabilization as benzene does. The situation get much more complicated with heterocyclic aromatic.
01-15-2006
Q: Problem 15.18 (c): The book gives the name of this structure as 1-bromo-3,5-dimethylbenzene. Is the name 3-bromo-5-methyltoluene also acceptable?
A: 3-bromo-5-methyltoluene is fine, although the non-systematic name for a dimethylbenzene is actually xylene (a meta-xylene in this case, although meta is not used in systematic nomenclature). When you start to get benzenes with more than two substituent and particularly if they are different, the names tend to become more systematic as in this case (1-bromo-3,5-dimethylbenzene) or if there can be an ambigutity over which parent name to use like in this case, toluene versus xylene. Had one of the methyl groups been a bromine, then 3,5-dibromotoulene would be correct.
Q: Problem 15.19 (a): Could this molecule also be named 1,2-diamino-3-methylbenzene? Problem 15.19 (b): Could this molecule also be named 1,3,5-trihydroxybenzene? What about 1,3,5-triphenol?
A: I actually object to the book’s use of 1,2-benzenediamine and 1,3,5-benzenetriol and would suggest 1,2-diamino-3-methylbenzene and 1,3,5-trihydroxybenzene be used. I would avoid using 1,3,5-triphenol; a phenol has a specific structural meaning and using the prefix tri is ambiguous. 3,5-dihydroxyphenol, on the other hand, is not ambiguous.
Q: Problem 15.39: The oxygen atom in the ring has two lone pairs of electrons. Do neither pair act in a p-orbital perpendicular to the plane of the ring in 4-pyrone? If not, why is this so. I pictured this to be like a nitrogen atom in which 1 lone pair of electrons contributes to the aromatic electrons whereas the other electrons are parallel to the plane of the ring (and in the case of nitrogen, bound to a hydrogen atom).
A: My feeling is that the ring oxygen of 4-pyrone is somewhat sp2 hybridized and one of the lone pairs is in fact in a p-orbital. You can draw a resonance structure of 4-pyrone in which there are three double bonds in the ring, a positive change on the ring oxygen, and a negative change on the other oxygen, which will have only a single bond (although it is still sp2 hybridized). This resonance structure is still overall charge neutral. Now how much does this resonance structure contribute to the overall structure? This is not necessarily an easy question to answer. It turns out the double bond of a carbonyl group (C=O) has a sizeable bond energy, so C=O’s are energetically quite favorable. It turns out the structure of 4-pyrone drawn in the book is the dominant resonance form. When 4-pyrone reacts with an acid, it acts as a Lewis base and the carbonyl oxygen accepts the proton (donates an electron pair to the proton). The resulting conjugate acid of 4-pyrone is has a resonance stabilized positive change. Because of this, you would expect 4-pyrone to be a stronger base than cyclohexanone.
Q: I have been working through these and noticed that each of the 13C NMR spectra has a peak at around 77 ppm. Looking at Figure 13.7 (page 432) of McMurry, I see that this can be an alkyne, a C-N, or C-O; however, not all the formulae given for these problems have those groups. Therefore, I was wondering what this absorption at 75-80 ppm is caused by?
A: The peak at 77 ppm is actually from (deuterated) chlorofom, the solvent.
Q: Will a reaction not occur if an anti-aromatic cation were to be produced? What does the acidity of cyclopentadiene have to do with it producing an aromatic anion in a reaction with a strong base?
A: A reaction that gives an antiaromatic product (cation, anion or uncharged) will be unfavorable. This does not mean that it won’t occur. A reaction that leads to an aromatic product is favorable. All reactions have thermodynamic parameters associated with them, i.e. an activation energy and a free energy change (DG, delta G). An aromatic product will make the DG of the reaction more favorable just as generating an antiaromatic product will make the reaction unfavorable. Treating cyclopentadiene with a base leads to a cyclopentadienyl anion, which is stabilized by aromaticity and is reflected in the pKa of cyclopentadiene (~15). Recall that the pKa is derived from the equilibrium between cyclopentadiene and base on reactant side and the cyclopentadienyl anion and conjugate acid of the base on the product side (see slide 21, and there should be equilibrium arrows for the reaction). Anything that stabilizes the product side of the equilibrium will shift the equilibrium toward products. While cyclopentadiene is still a weak acid (it has about the same pKa as water), it is much more acidic than most hydrocarbons, which is attributed to the aromatic stabilization of cyclopentadienyl anion.
Q: Do we have to know the structures of PAH’s you had on the slide?
A: You do not need to know the structures of the PAH except for naphthalene.
Q: Are the other problems in the back of the chapter besides the recommended problems not as relevant to what we are learning (i.e. should we know how to answer those problems too??
A: I recommend that you do as many problems as you have time for. The problems that I list are typical of thing that you should know. Some of the problems get redundant so I don’t list them if I have recommended others that were similar. If I skip a section, I won’t recommend problems from skipped sections.
Q: Problem 15.2f, is 2,3,5-trimethyltoluene the same as 1,2,3,5-tetramethylbenzene?
A: 1,2,3,5-tetramethylbenzene is actually the correct name although 2,3,5-trimethyltoluene is not ambiguous. When you start to get into benzenes with more than two substituents and particularly if they are different, the names tend to become more systematic as in this case. One problem with using toluene as the parent name is that a benzene with two methyl groups is actually called a xylene. One reason that I do not like to test specifically on nomenclature is that the rules are quite tedious. However, it is necessary to be acquainted with nomenclature to the extent that you know what is going on. Above all else, I want to avoid sloppy nomenclature that lead to ambiguities or
misunderstandings.
Q: In a molecule like indole, how do you know when nitrogen’s two electrons will be planar or perpendicular and thus make the molecule aromatic?
A: If the lone pair on nitrogen can make a molecule aromatic, as in the case of indole and pyrrole, it will. As noted above, the aromaticity is a stabilizing feature and all molecules or chemical reactions will achieve their lowest energy state.
Q: In problem 15.18a, the correct answer is 2-methyl-5-phenylhexane, but I thought that only when the side chain has more than 6 carbons it becomes the parent chain. Here, they’ve made a hexane the parent chain over the benzene. How?
A: It looks as though I may have mis-interpreted the rule for naming an alkylbenzene. I will look to consult another source. It may be that you look at the total number of carbons of the alkyl group rather than the longest chain of the alkyl group (as I may have incorrectly stated). For 15.18a, there are actually seven carbons on the alkyl group giving it priority over the benzene ring.