Dean of Research David Wright Explains Coronavirus Facts on Alumni Webinar
More than 500 College of Arts and Science alumni registered for an April 22 coronavirus webinar with David Wright, Stevenson Professor of Chemistry, Dean of Graduate Education & Research, and expert on respiratory viruses and diagnostics. The webinar, which was hosted by the Office of Development and Alumni Relations, opened with remarks from John Geer, the Ginny and Conner Searcy Dean of the College of Arts and Science.
“One of the things the College is really good at is tackling the big issues that confront society, and we have a pretty big one in our midst,” Geer said.

Wright began by outlining his own research experience and the work he is doing now in the fight against COVID-19. Wright has done extensive research on malaria, effective methods for diagnosing it, and managing the public health crises it tends to create. Through that experience, he’s learned how to develop effective tests and public health plans that could be useful in our current crisis. In countries such as Zambia, where trained medical staff, clean water, lab equipment, and electricity are scarce or unreliable, virus tests must be cheap to manufacture and distribute, easy for untrained people to administer, and quick to provide results. These are all qualities we need now for coronavirus tests, which are currently in scarce supply and take 3 to 14 days to process—too long to be useful in determining the need for interventions such as self-isolation or contact tracing.
“The diagnostic testing problem is one of the biggest challenges we’re facing in opening up the country,” Wright said. “We can’t make decisions based on things we don’t know.”
To help bridge the gap while Wright and other researchers develop new tests, Wright’s lab has created a virtual screening tool that helps individuals determine in a matter of minutes whether they might have COVID-19. The app uses a self-assessment based on the CDC’s COVID-19 diagnostic guidelines and points users to nearby testing centers with immediate availability.
The Origins of Coronavirus and Vaccine Development
Wright also addressed a number of audience questions, submitted both before and during the webinar. One set of questions concerned the novel coronavirus’s origins and vaccine development.
According to Wright, scientists generally agree that the current virus likely passed from a bat, which is a common host for coronaviruses in the wild, to a pangolin that was sold as food in a market in Wuhan, China. A person who came in contact with the pangolin after it was infected, or who ate the pangolin, was likely the first human host. Wright explained that this transmission pattern is common for other viruses—such as Ebola and HIV—that have passed from wild animals to humans.
Genetic testing conducted by the University of Washington has shown that the coronavirus strains infecting people all over the world evolved from one source: the virus that originated in Wuhan. The same tests also show that the virus does not often mutate in areas where antibodies would normally attach to it—a promising sign for vaccine researchers. This mutation pattern, plus the fact that the virus strains come from a single source, mean that once a vaccine is developed it will likely be effective and long-lasting.
Though having a vaccine widely available before summer 2021 “would be a world record,” Wright said, the virus’s stability “is one of the things that works in our favor as we face the days ahead. We might only have to rework the vaccine every three to five years.”
Testing and Immunity
Participants also submitted several questions about testing and immunity. There are two types of coronavirus tests currently in circulation, Wright explained: tests that check for the virus itself (diagnostic tests) and tests that check for antibodies left behind after infection. According to Wright, both are essential. Identifying active infection is currently the top priority, to help reduce the virus’s spread. Immunity testing will be most useful as social restrictions ease, to help determine how people can safely return to work and public activities.
Existing diagnostic tests are highly accurate, said Wright. But because there are not enough test kits in circulation to enable widespread testing—a necessary step for safely reopening the country—getting new tests to market is crucial. The CDC is therefore allowing reduced red tape for trusted labs, like Wright’s, that are seeking approval for new tests they’ve developed.
Some labs are trying to solve a second problem: the fallibility of antibody tests. Existing antibody tests, Wright explained, are good at distinguishing coronavirus antibodies from other types but are not very sensitive to low concentrations of the antibodies. This means people may test negative for antibodies when they actually have them. The second generation of tests, Wright said, will hopefully solve this problem.
Once enough people have antibodies, the U.S. will achieve herd immunity: the point at which the virus can no longer find enough new hosts to stay alive in the population. Because coronavirus is highly contagious, however, herd immunity requires immunity in roughly 70 percent of the population. (Herd immunity to measles, by comparison, requires a population immunity rate of 90 percent. For seasonal flu, the rate is 40 percent.)
“Without a vaccine, that’s simply not possible,” Wright said. “Seventy percent of 300 million people would have to become infected, which would lead to millions of deaths. Until we have an effective vaccine, it’s hard to imagine we’ll get herd immunity.”
How to Win the Fight
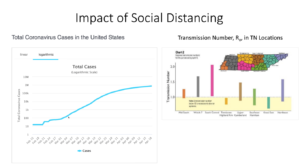
As Wright’s numbers indicate, the coronavirus threat is severe. For most people, seasonal flu is the most familiar point of comparison. But coronavirus is far more dangerous, for several reasons.
First, Wright explained, the virus is roughly twice as contagious as the flu. While one flu carrier typically infects 1.1-1.4 additional people, a coronavirus carrier typically infects 3. Second, COVID-19 causes life-threatening complications that are not typically a risk with flu: hypoxia (chronic oxygen deprivation), multiple organ failure, and systemic blood clots that lead to stroke, heart attack, or limb amputation. Third, we don’t yet have a vaccine or proven, widely-available treatments for COVID-19. Antibody therapy is effective, but the demand for it is roughly double the supply. The anti-malarial drug hydroxychloroquine has been tested and proven ineffective. And the antiviral drug remdesivir, which looks promising, is still undergoing testing and has been approved on an emergency basis only.
These factors, Wright explained, are what increase the risk that our healthcare system may become overwhelmed with COVID-19 patients. They are also why social distancing is so important until infection rates slow and we have effective treatments or a vaccine.
“In Tennessee, the counties with the best social distancing have the lowest transmission numbers,” Wright explained. He referenced data showing a discernible “flattening of the curve” (decrease in transmission rates) beginning March 19, soon after social distancing took effect in most areas of the U.S.
“There’s never been a time when it’s been more important to treat other people with the kindness and the humanity we want to be treated with,” Wright said. “Science will provide the tools, and changing social behaviors will provide the human part of the equation that we need to solve this problem.”
